NDC Code(s) : 0944-0491-01, 0944-0491-02
Packager : Baxalta US Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BUMINATEAlbumin Human INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
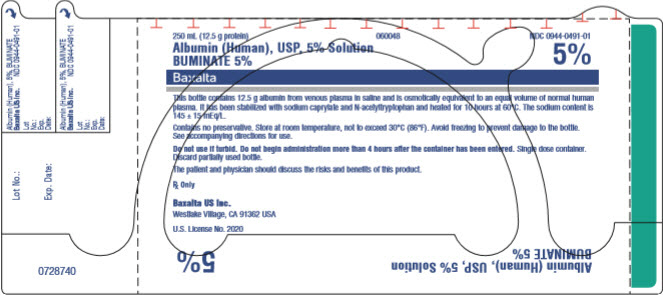 Buminate 5% 250 mL bottle label
Buminate 5% 250 mL bottle label
Buminate 5% 250 mL bottle label
250 mL (12.5g protein)
NDC 0944-0491-01
Albumin (Human), USP, 5% Solution
BUMINATE 5%
This bottle contains 12.5 g albumin from venous plasma in saline and is osmotically equivalent to an equal volume of normal human plasma. It has been stabilized with sodium caprylate and N-acetyltryptophan and heated for 10 hours at 60°C. The sodium content is 145 ± 15 mEq/L.
Contains no preservative. Store at room temperature, not to exceed 30°C (86°F). Avoid freezing to prevent damage to the bottle. See accompanying directions for use.
Do not use if turbid. Do not begin administration more than 4 hours after the container has been entered. Single dose container. Discard partially used bottle.
The patient and physician should discuss the risks and benefits of this product.
Rx Only
Baxalta US Inc.
Westlake Village, CA 91362 USA
US. License No. 2020
PRINCIPAL DISPLAY PANEL
 Buminate 5% 500 mL bottle label
Buminate 5% 500 mL bottle label
Buminate 5% 500 mL bottle label
500 mL (25 g protein)
NDC 0944-0491-02
Albumin (Human), USP, 5% Solution
BUMINATE 5%
This bottle contains 25g albumin from venous plasma in saline and is osmotically equivalent to an equal volume of normal human plasma. It has been stabilized with sodium caprylate and N-acetyltryptophan and heated for 10 hours at 60ºC. The sodium content is 145 ± 15 mEq/L. Contains no preservative. Store at room temperature, not to exceed 30ºC (86ºF). Avoid freezing to prevent damage to the bottle. See accompanying directions for use.
Do not use if turbid. Do not begin administration more than 4 hours after the container has been entered. Single dose container. Discard partially used bottle.
The patient and physician should discuss the risks and benefits of this product.
Rx Only
Baxalta US Inc.
Westlake Village, CA 91362 USA
US. License No. 2020









