NDC Code(s) : 0548-1190-00, 0548-2190-00, 0548-3390-00
Packager : Amphastar Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Lidocaine Hydrochloride Lidocaine Hydrochloride INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lidocaine Hydrochloride Lidocaine Hydrochloride INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lidocaine Hydrochloride Lidocaine Hydrochloride INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
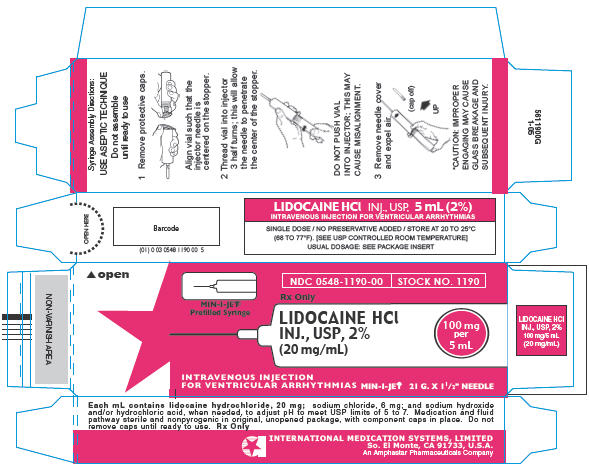
PRINCIPAL DISPLAY PANEL
NDC 0548-1190-00
STOCK NO. 1190
Rx Only
MIN-I-JET®
Prefilled Syringe
LIDOCAINE HCl
INJ., USP, 2%
(20 mg/mL)
100 mg
per
5 mL
INTRAVENOUS INJECTION
FOR VENTRICULAR ARRHYTHMIAS MIN-I-JET® 21 G. X 1½" NEEDLE
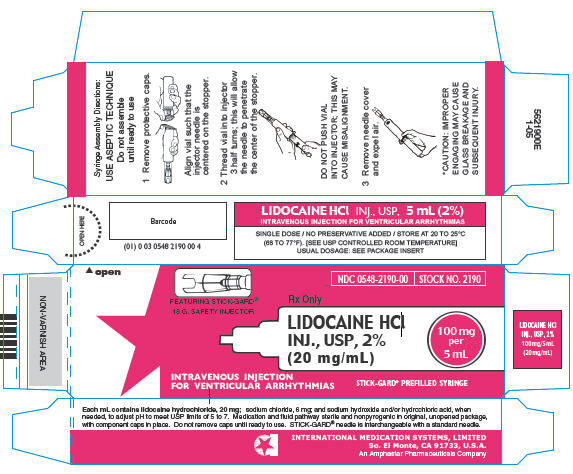
PRINCIPAL DISPLAY PANEL
NDC 0548-2190-00
STOCK NO. 2190
Rx Only
FEATURING STICK-GARD®
18 G. SAFETY INJECTOR
LIDOCAINE HCl
INJ., USP, 2%
(20 mg/mL)
100 mg
per
5 mL
INTRAVENOUS INJECTION
FOR VENTRICULAR ARRHYTHMIAS
STICK-GARD® PREFILLED SYRINGE
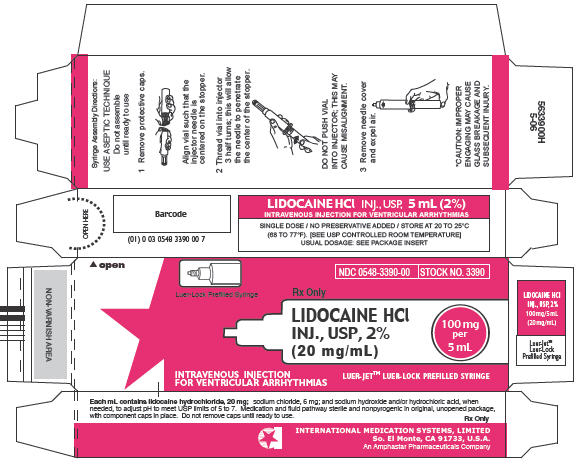
PRINCIPAL DISPLAY PANEL
NDC 0548-3390-00
STOCK NO. 3390
Rx Only
Luer-Lock Prefilled Syringe
LIDOCAINE HCl
INJ., USP, 2%
(20 mg/mL)
100 mg
per
5 mL
INTRAVENOUS INJECTION
FOR VENTRICULAR ARRHYTHMIAS
LUER-JET™ LUER-LOCK PREFILLED SYRINGE