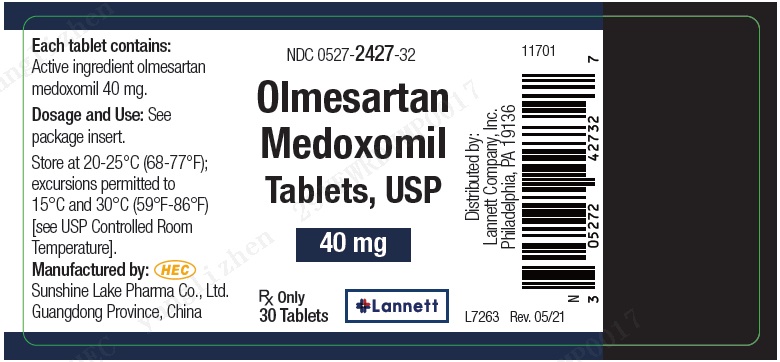
NDC Code(s) : 0527-2425-32, 0527-2425-46, 0527-2426-32, 0527-2426-46, 0527-2427-32, 0527-2427-46
Packager : Lannett Company, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| OLMESARTAN MEDOXOMILOlmesartan medoxomil TABLET, FILM COATED | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| OLMESARTAN MEDOXOMILOlmesartan medoxomil TABLET, FILM COATED | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| OLMESARTAN MEDOXOMILOlmesartan medoxomil TABLET, FILM COATED | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Lannett Company, Inc.(002277481) |
| REGISTRANT - Sunshine Lake Pharma Co., Ltd.(545391443) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Sunshine Lake Pharma Co., Ltd. | 545391443 | manufacture(0527-2425, 0527-2426, 0527-2427) | |
PRINCIPAL DISPLAY PANEL
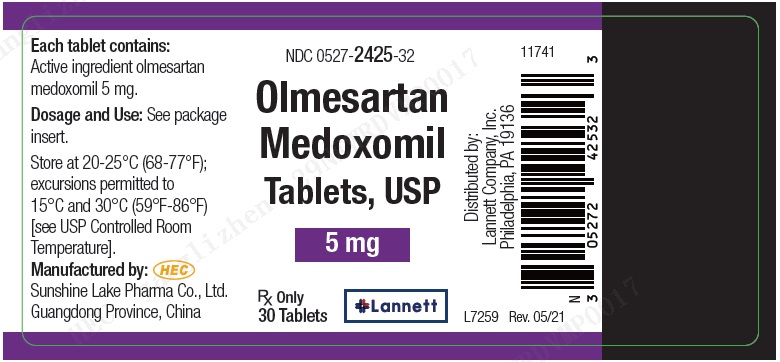
PRINCIPAL DISPLAY PANEL
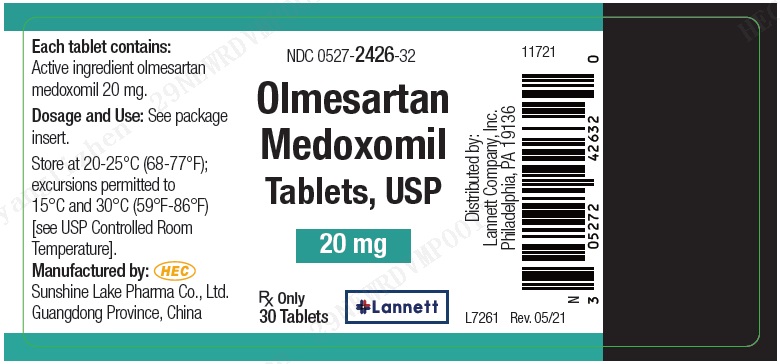
PRINCIPAL DISPLAY PANEL