NDC Code(s) : 0462-0358-15, 0462-0358-30, 0462-0358-60, 0462-0359-30, 0462-0359-60
Packager : PharmaDerm, A division of Nycomed US Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Oxistatoxiconazole nitrate CREAM | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Oxistatoxiconazole nitrate LOTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
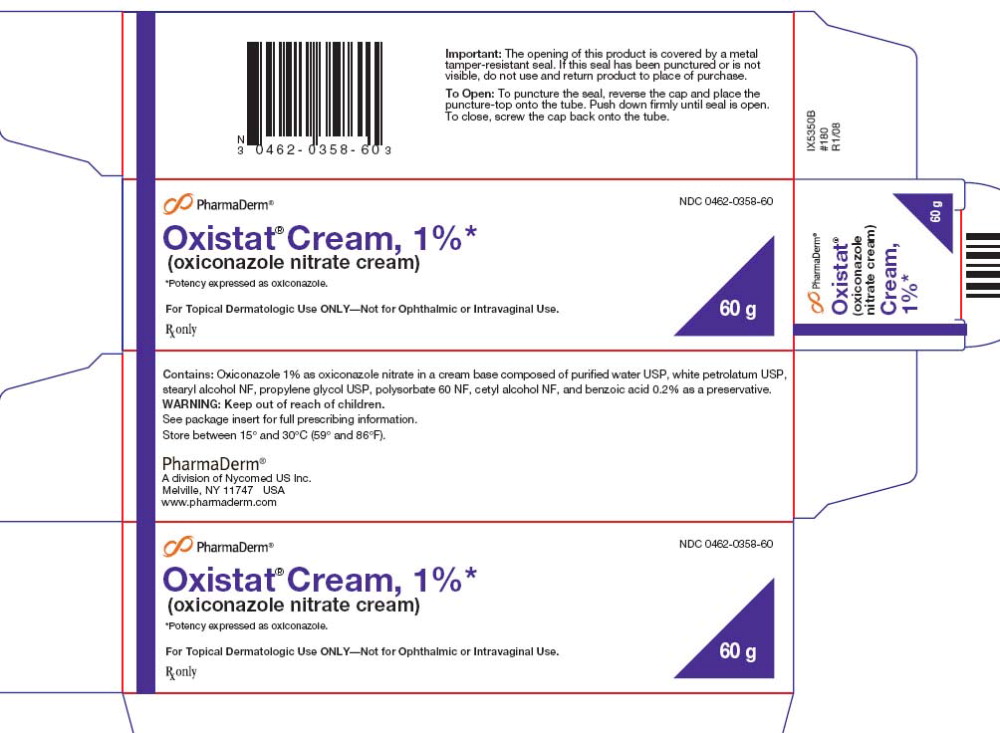
PRINCIPAL DISPLAY PANEL
PharmaDerm®
NDC 0462-0358-60
Oxistat® Cream, 1%*
(oxiconazole nitrate cream)
*Potency expressed as oxiconazole.
For Topical Dermatologic Use ONLY – Not for Ophthalmic or Intravaginal Use.
Rx only
60 g
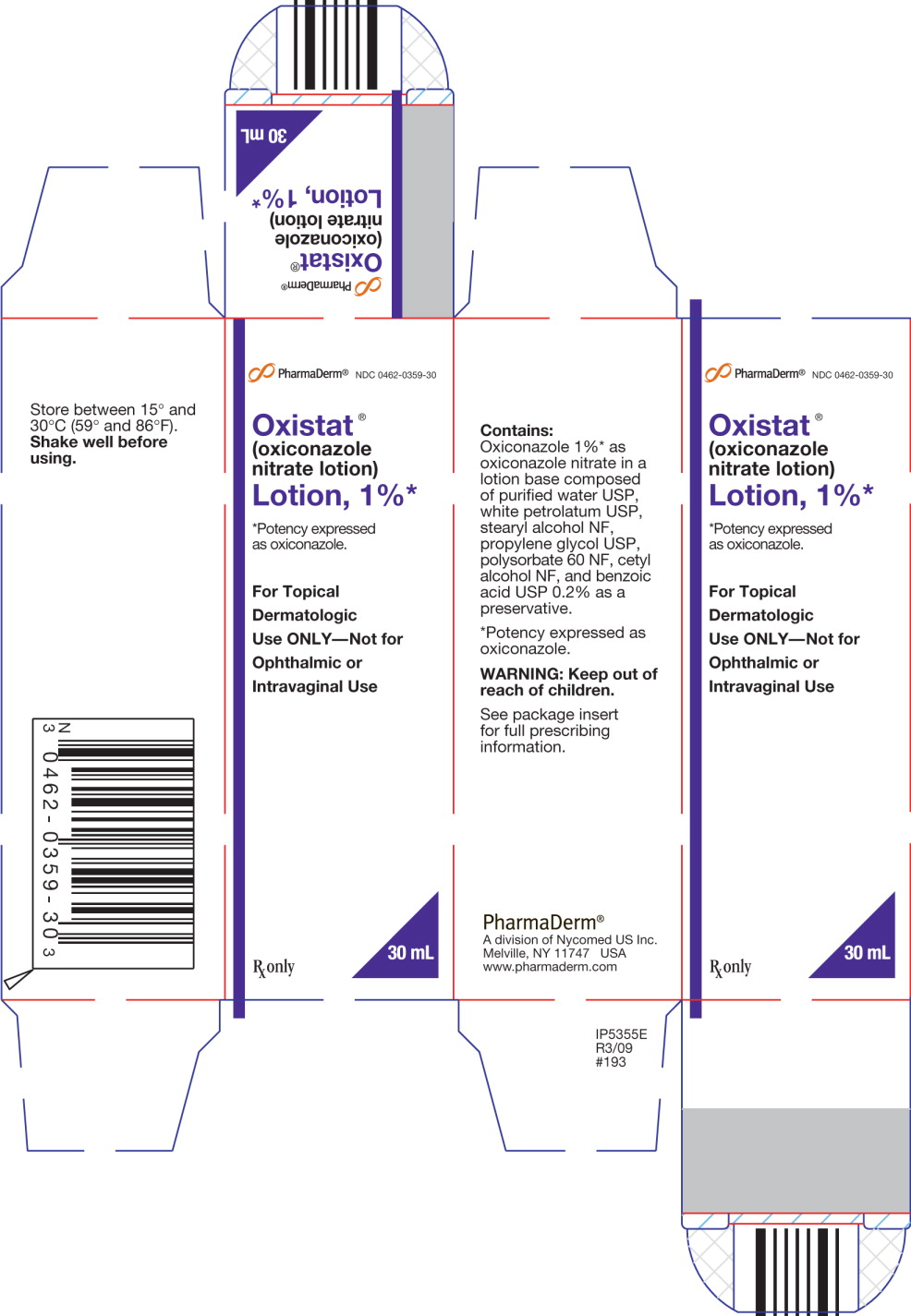
PRINCIPAL DISPLAY PANEL
PharmaDerm®
NDC 0462-0359-30
Oxistat®
(oxiconazole nitrate lotion)
Lotion, 1%*
*Potency expressed as oxiconazole.
For Topical Dermatologic Use ONLY – Not for Ophthalmic or Intravaginal Use
Rx only
30 mL