NDC Code(s) : 0409-7337-21, 0409-7337-20, 0409-7338-21, 0409-7338-20, 0409-7332-21, 0409-7332-20, 0409-7335-21, 0409-7335-20
Packager : Hospira, Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Ceftriaxone SodiumCeftriaxone Sodium INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Ceftriaxone SodiumCeftriaxone Sodium INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Ceftriaxone SodiumCeftriaxone Sodium INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Ceftriaxone SodiumCeftriaxone Sodium INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Hospira, Inc(141588017) |
PRINCIPAL DISPLAY PANEL
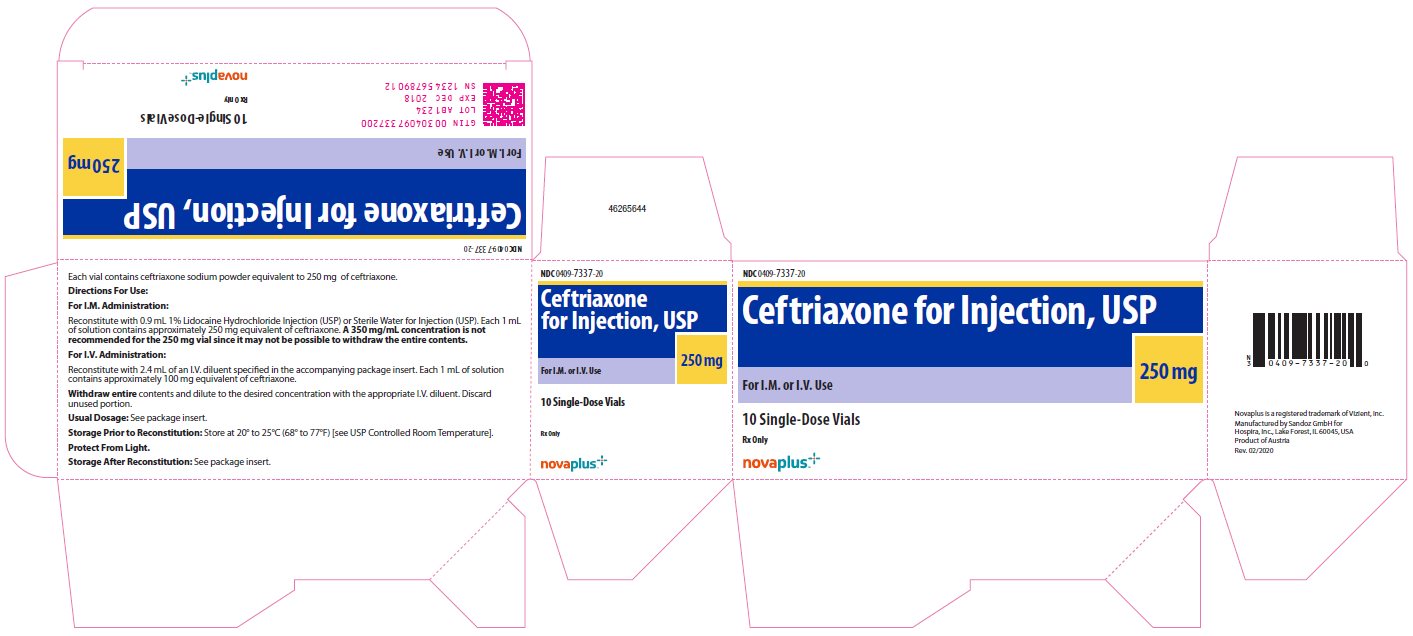
NDC 0409-7337-20
Ceftriaxone
for Injection, USP
250 mg
For I.M. or I.V. Use
10 Single-Dose Vials
Rx Only
novaplus
PRINCIPAL DISPLAY PANEL

- NDC 0409-7338-20
Ceftriaxone
for Injection, USP
500 mg
For I.M. or I.V. Use
10 Single-Dose Vials
Rx Only
novaplus
PRINCIPAL DISPLAY PANEL
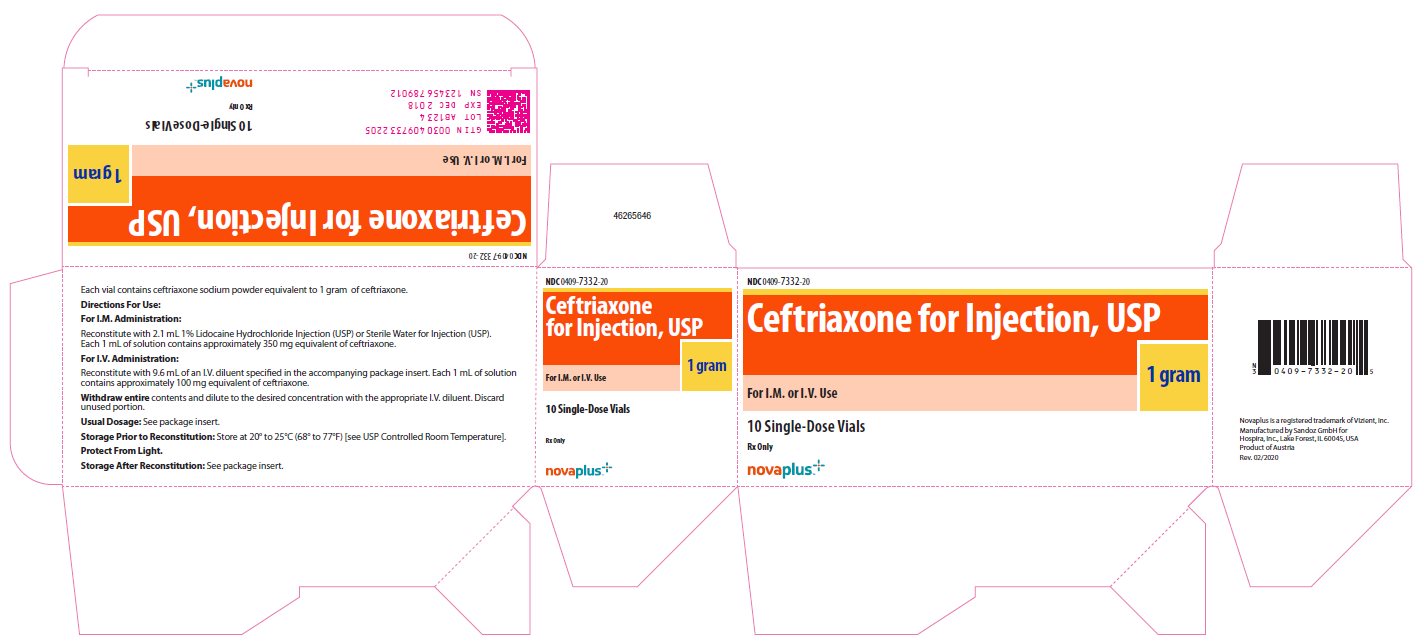
- NDC 0409-7332-20
Ceftriaxone
for Injection, USP
1 gram
For I.M. or I.V. Use
10 Single-Dose Vials
Rx Only
novaplus
PRINCIPAL DISPLAY PANEL
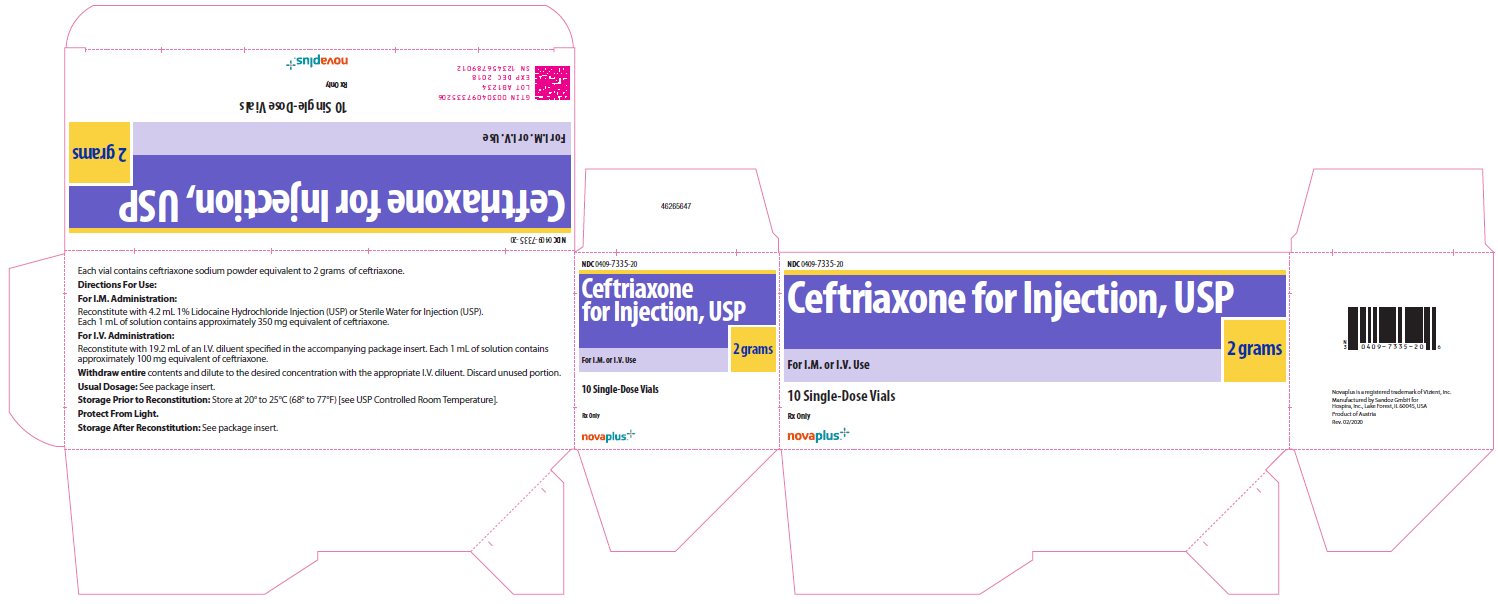
NDC 0409-7335-20
Ceftriaxone
for Injection, USP
2 grams
For I.M. or I.V. Use
10 Single-Dose Vials
Rx Only
novaplus









