NDC Code(s) : 0378-0825-01, 0378-0825-02, 0378-0972-01, 0378-0972-02, 0378-0860-01, 0378-0860-05, 0378-0860-02, 0378-0860-07, 0378-0973-01, 0378-0973-02
Packager : Mylan Pharmaceuticals Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Clozapineclozapine TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Clozapineclozapine TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Clozapineclozapine TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Clozapineclozapine TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Mylan Pharmaceuticals Inc.(059295980) |
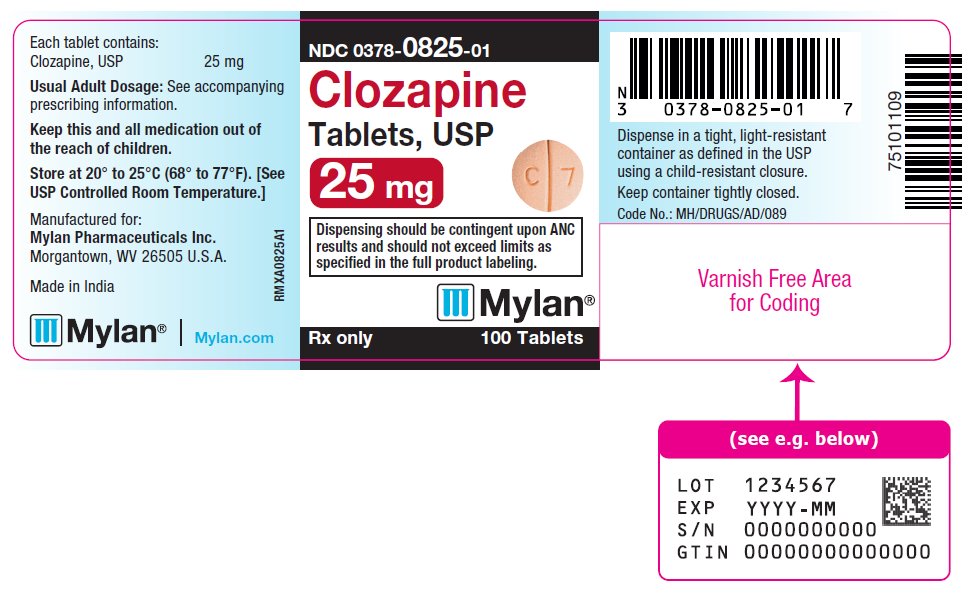
PRINCIPAL DISPLAY PANEL
NDC 0378-0825-01
Clozapine
Tablets, USP
25 mg
Dispensing should be contingent upon ANC
results and should not exceed limits as
specified in the full product labeling.
Rx only 100 Tablets
Each tablet contains:
Clozapine, USP 25 mg
Usual Adult Dosage: See accompanying
prescribing information.
Keep this and all medication out of
the reach of children.
Store at 20° to 25°C (68° to 77°F). [See
USP Controlled Room Temperature.]
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Made in India
Mylan.com
RMXA0825A1
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Code No.: MH/DRUGS/AD/089
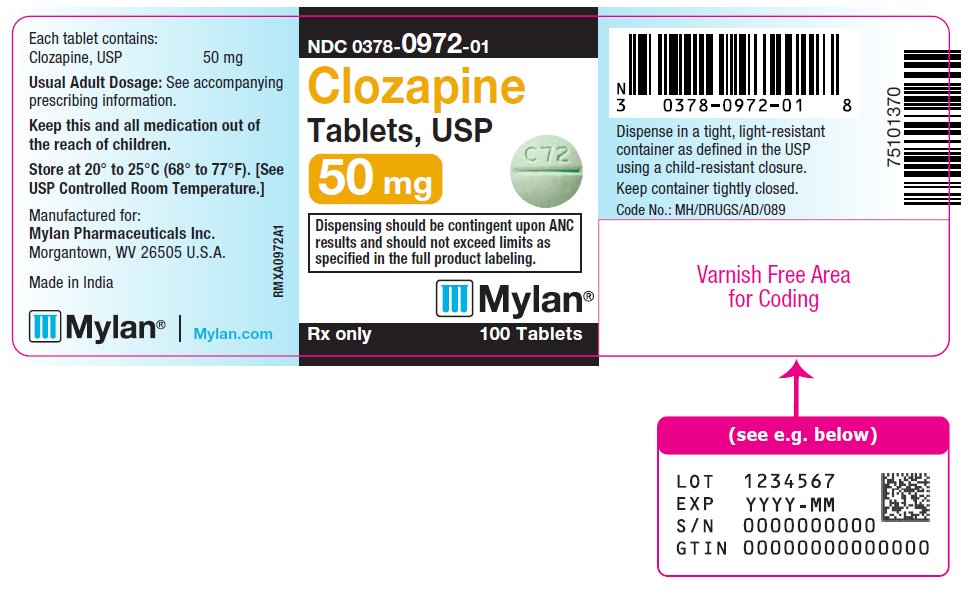
PRINCIPAL DISPLAY PANEL
NDC 0378-0972-01
Clozapine
Tablets, USP
50 mg
Dispensing should be contingent upon ANC
results and should not exceed limits as
specified in the full product labeling.
Rx only 100 Tablets
Each tablet contains:
Clozapine, USP 50 mg
Usual Adult Dosage: See accompanying
prescribing information.
Keep this and all medication out of
the reach of children.
Store at 20° to 25°C (68° to 77°F). [See
USP Controlled Room Temperature.]
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Made in India
Mylan.com
RMXA0972A1
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Code No.: MH/DRUGS/AD/089
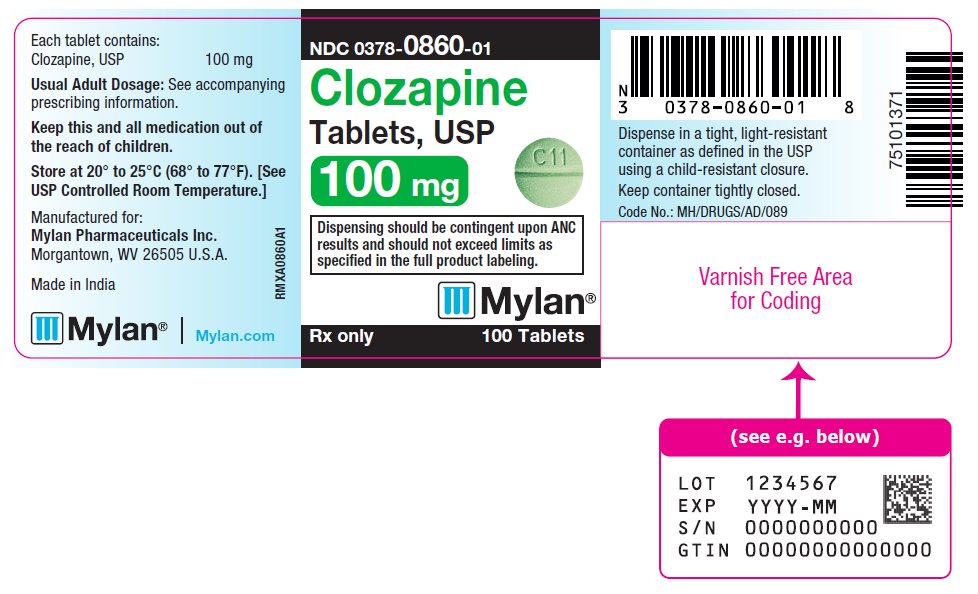
PRINCIPAL DISPLAY PANEL
NDC 0378-0860-01
Clozapine
Tablets, USP
100 mg
Dispensing should be contingent upon ANC
results and should not exceed limits as
specified in the full product labeling.
Rx only 100 Tablets
Each tablet contains:
Clozapine, USP 100 mg
Usual Adult Dosage: See accompanying
prescribing information.
Keep this and all medication out of
the reach of children.
Store at 20° to 25°C (68° to 77°F). [See
USP Controlled Room Temperature.]
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Made in India
Mylan.com
RMXA0860A1
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Code No.: MH/DRUGS/AD/089
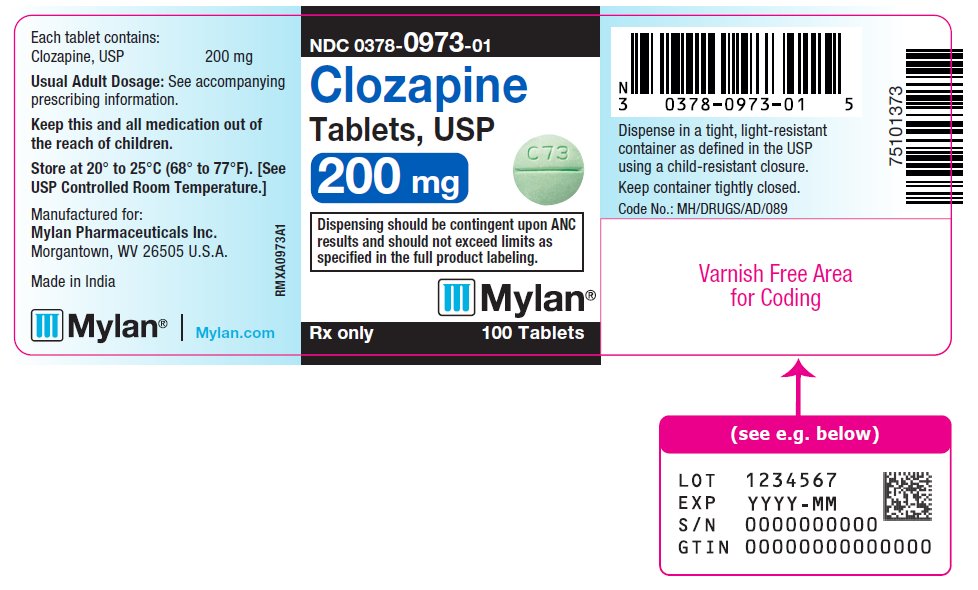
PRINCIPAL DISPLAY PANEL
NDC 0378-0973-01
Clozapine
Tablets, USP
200 mg
Dispensing should be contingent upon ANC
results and should not exceed limits as
specified in the full product labeling.
Rx only 100 Tablets
Each tablet contains:
Clozapine, USP 200 mg
Usual Adult Dosage: See accompanying
prescribing information.
Keep this and all medication out of
the reach of children.
Store at 20° to 25°C (68° to 77°F). [See
USP Controlled Room Temperature.]
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Made in India
Mylan.com
RMXA0973A1
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Code No.: MH/DRUGS/AD/089