NDC Code(s) : 0338-9051-01, 0338-9051-12, 0338-9053-01, 0338-9053-12, 0338-9055-01, 0338-9055-10
Packager : Baxter Healthcare Corporation
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Micafungin in Sodium ChlorideMicafungin in Sodium Chloride INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Micafungin in Sodium ChlorideMicafungin in Sodium Chloride INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Micafungin in Sodium ChlorideMicafungin in Sodium Chloride INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Baxter Healthcare Corporation(005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Baxter Healthcare Corporation | 194684502 | ANALYSIS(0338-9051, 0338-9053, 0338-9055), LABEL(0338-9051, 0338-9053, 0338-9055), MANUFACTURE(0338-9051, 0338-9053, 0338-9055), PACK(0338-9051, 0338-9053, 0338-9055), STERILIZE(0338-9051, 0338-9053, 0338-9055) | |
PRINCIPAL DISPLAY PANEL

 Container Label
Container Label
NDC 0338-9051-01
Micafungin
in 0.9% Sodium Chloride Injection
50 mg / 50 mL (1 mg / mL)
50 mg
TOTAL
50 mL Single-Dose GALAXY container
Discard unused portion
For Intravenous Infusion Only
Rx only
Sterile Nonpyrogenic
Cautions: Do not add supplemental medication or additives.
Must not be used in series connections. Check for minute leaks and solution
clarity.
Each 50 mL bag contains: 50 mg of Micafungin Sodium (equivalent to 50.87 mg of
Micafungin Sodium); 36 mg Citric Acid, Anhydrous; 450 mg Sodium Chloride,
92 mg Sodium Citrate, Dihydrate; and Water for Injection.
Dosage: See prescribing information.
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to
protect from light. Do Not Freeze.
If needed, may store at room temperature up to 25°C (77°F) up to 30 days in
the original carton. Discard after 30 days if stored at room temperature.
Baxter Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015 USA
Code 2G3434
PL 2501 Plastic
Product of USA
07-34-00-1662
BAR CODE
POSITION ONLY UPCA-A
XXXXXXXXXXXX

 Container Label
Container Label
NDC 0338-9053-01
Micafungin
in 0.9% Sodium Chloride Injection
100 mg / 100 mL (1 mg / mL)
100 mg
TOTAL
100 mL Single-Dose GALAXY container
Discard unused portion
For Intravenous Infusion Only
Rx only
Sterile Nonpyrogenic
Cautions: Do not add supplemental medication or additives.
Must not be used in series connections. Check for minute leaks
and solution clarity.
Each mL contains: 100 mg of Micafungin (equivalent
to 101.73 mg of Micafungin Sodium); 72 mg Citric Acid,
Anhydrous; 900 mg Sodium Chloride; 184 mg Sodium Citrate,
Dihydrate; and Water for Injection.
Dosage: See prescribing information.
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original
carton to protect from light. Do Not Freeze.
If needed, may store at room temperature up to 25°C (77°F) up
to 30 days in the original carton. Discard after 30 days if stored at
room temperature.
Baxter Logo
Baxter and Galaxy are registered trademarks of
Baxter International Inc.
Baxter Healthcare Corporation,
Deerfield, IL 60015 USA
Code 2G3435
PL 2501 Plastic
Product of USA
07-34-00-1663
BAR CODE
POSITION ONLY UPCA-A
XXXXXXXXXXXX

 Container Label
Container Label
NDC 0338-9055-01
Micafungin
in 0.9% Sodium Chloride Injection
150 mg / 150 mL (1 mg / mL)
150
mg
TOTAL
150 mL Single-Dose GALAXY container
Discard unused portion
For Intravenous Infusion Only
Rx only
Sterile Nonpyrogenic
Cautions: Do not add supplemental medication or additives.
Must not be used in series connections. Check for minute leaks
and solution clarity.
Each mL contains: 150 mg of Micafungin (equivalent
to 152.60 mg of Micafungin Sodium); 108 mg Citric Acid,
Anhydrous; 1350 mg Sodium Chloride; 276 mg Sodium Citrate,
Dihydrate; and Water for Injection.
Dosage: See prescribing information.
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original
carton to protect from light. Do Not Freeze.
If needed, may store at room temperature up to 25°F (77°F) up
to 30 days in the original carton. Discard after 30 days if stored at
room temperature.
Baxter Logo
Baxter and Galaxy are registered trademarks of
Baxter International Inc.
Baxter Healthcare Corporation,
Deerfield, IL 60015 USA
Code 2G3433
PL 2501 Plastic
Product of USA
07-34-00-1664
BAR CODE
POSITION ONLY UPCA-A
XXXXXXXXXXXX
 Carton Label
Carton Label
For Intravenous Only
Micafungin
in 0.9% Sodium Chloride Injection
50 mg per 50 mL (1 mg / mL)
For Intravenous Only
Micafungin
in 0.9% Sodium Chloride Injection
50 mg per 50 mL (1 mg / mL)
NDC 0338-9051-01
Sterile, Nonpyrogenic
Micafungin
in 0.9% Sodium Chloride Injection
50 mg / 50 mL (1 mg / mL)
50 mg
TOTAL
UNVARNISHED AREA FOR ON-LINE
PRINTING OF LOT & EXP
FPO UPC-A Barcode
085412XXXXXX
Each 50 mL bag contains: 50 mg of Micafungin (equivalent to 50.87 mg of Micafungin Sodium);
36 mg Citric Acid, Anhydrous; 450 mg Sodium Chloride; 92 mg Sodium Citrate, Dihydrate;
and Water for Injection.
Dosage: For Intravenous Infusion Only. See prescribing information.
Caution: Do not add supplemental medication or additives.
RX only
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
Do Not Freeze
If needed, may store at room temperature up to 25°C (77°F) up to 30 days in the original carton.
Once stored at room temperature, do not place back in the refrigerator.
Discard after 30 days if stored at room temperature
Discard 30 days after storing at room temperature. Discard after:
(Month) (Day) (Year)
(to be completed by dispensing pharmacist)
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Product of USA
07-01-00-0593
For Intravenous Infusion Only
Micafungin
in 0.9% Sodium Chloride Injection
50 mg per 50 mL (1 mg / mL)
For Intravenous Infusion Only
Micafungin
in 0.9% Sodium Chloride Injection
50 mg per 50 mL (1 mg / mL)
NDC 0338-9051-01
Code 2G3434
Micafungin
in 0.9% Sodium Chloride Injection
50 mg / 50 mL (1 mg / 1 mL)
50 mg
TOTAL
Rx Only
For Intravenous Infusion Only
1 Single-Dose GALAXY Container
Discard unused portion
USE CARTON TO PROECT CONTENTS
FROM LIGHT UNTIL ADMINISTRATION
Baxter Logo
 Carton Label
Carton Label
For Intravenous Only
Micafungin
in 0.9% Sodium Chloride Injection
100 mg per 100 mL (1 mg / mL)
For Intravenous Only
Micafungin
in 0.9% Sodium Chloride Injection
100 mg per 100 mL (1 mg / mL)
NDC 0338-9053-01
Sterile, Nonpyrogenic
Micafungin
in 0.9% Sodium Chloride Injection
100 mg / 100 mL (1 mg / mL)
100 mg
TOTAL
UNVARNISHED AREA FOR ON-LINE
PRINTING OF LOT & EXP
FPO UPC-A Barcode
085412XXXXXX
Each 100 mL bag contains: 100 mg of Micafungin (equivalent to 101.73 mg of Micafungin Sodium);
72 mg Citric Acid, Anhydrous; 900 mg Sodium Chloride; 184 mg Sodium Citrate, Dihydrate;
and Water for Injection.
Dosage: For Intravenous Infusion Only. See prescribing information.
Caution: Do not add supplemental medication or additives.
RX only
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
Do Not Freeze
If needed, may store at room temperature up to 25°C (77°F) up to 30 days in the original carton.
Once stored at room temperature, do not place back in the refrigerator.
Discard after 30 days if stored at room temperature
Discard 30 days after storing at room temperature. Discard after:
(Month) (Day) (Year)
(to be completed by dispensing pharmacist)
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Product of USA
07-01-00-0613
For Intravenous Infusion Only
Micafungin
in 0.9% Sodium Chloride Injection
100 mg per 100 mL (1 mg / mL)
For Intravenous Infusion Only
Micafungin
in 0.9% Sodium Chloride Injection
100 mg per 100 mL (1 mg / mL)
NDC 0338-9053-01
Code 2G3435
Micafungin
in 0.9% Sodium Chloride Injection
100 mg / 100 mL (1 mg / 1 mL)
100 mg
TOTAL
Rx Only
For Intravenous Infusion Only
1 Single-Dose GALAXY Container
Discard unused portion
USE CARTON TO PROECT CONTENTS
FROM LIGHT UNTIL ADMINISTRATION
Baxter Logo
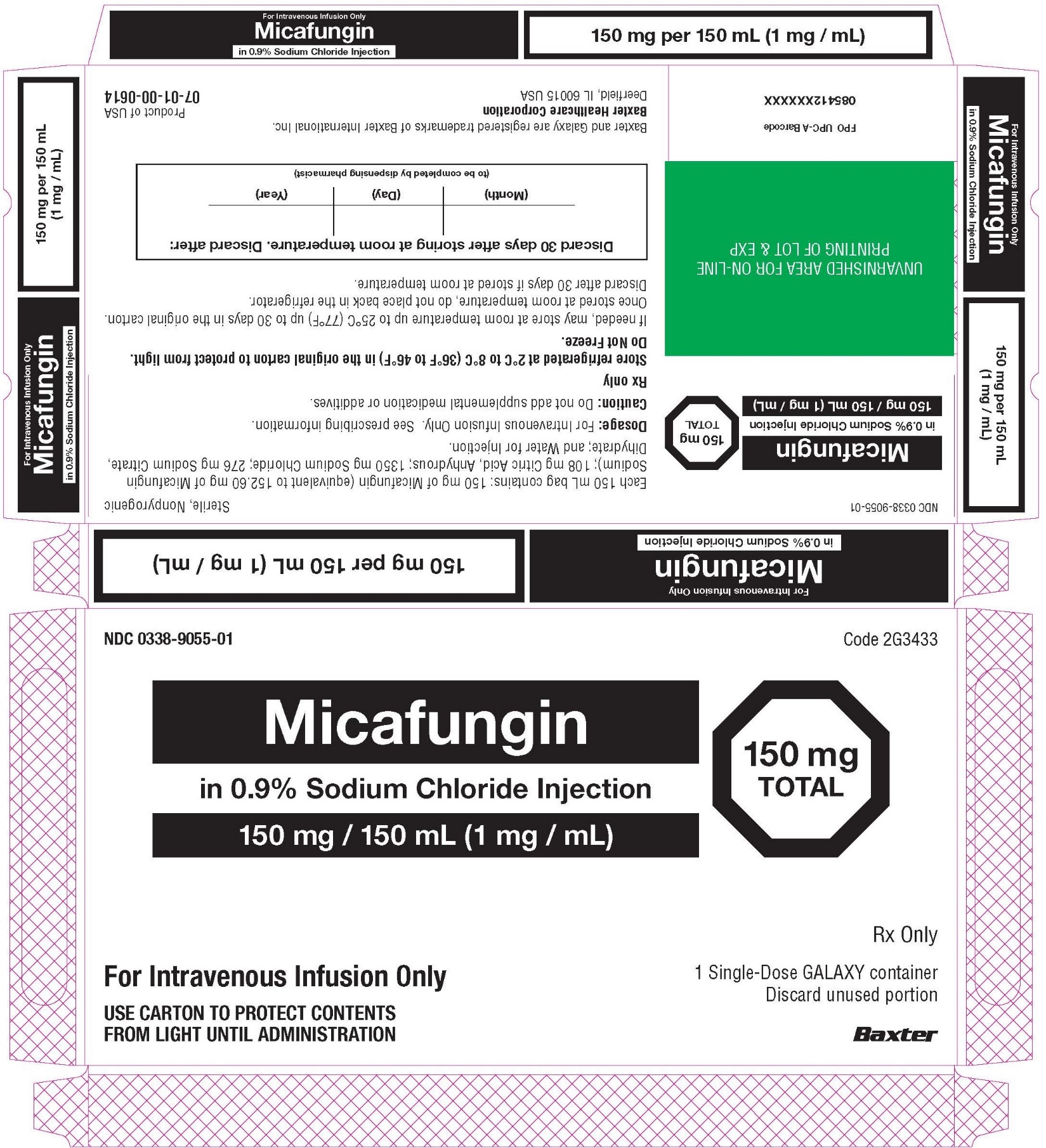 Carton Label
Carton Label
For Intravenous Only
Micafungin
in 0.9% Sodium Chloride Injection
150 mg per 150 mL (1 mg / mL)
For Intravenous Only
Micafungin
in 0.9% Sodium Chloride Injection
150 mg per 150 mL (1 mg / mL)
NDC 0338-9055-01
Sterile, Nonpyrogenic
Micafungin
in 0.9% Sodium Chloride Injection
150 mg / 150 mL (1 mg / mL)
150 mg
TOTAL
UNVARNISHED AREA FOR ON-LINE
PRINTING OF LOT & EXP
FPO UPC-A Barcode
085412XXXXXX
Each 150 mL bag contains: 150 mg of Micafungin (equivalent to 152.60 mg of Micafungin
Sodium); 108 mg Citric Acid, Anhydrous; 1350 mg Sodium Chloride; 276 mg Sodium Citrate,
Dihydrate; and Water for Injection.
Dosage: For Intravenous Infusion Only. See prescribing information.
Caution: Do not add supplemental medication or additives.
RX only
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
Do Not Freeze
If needed, may store at room temperature up to 25°C (77°F) up to 30 days in the original carton.
Once stored at room temperature, do not place back in the refrigerator.
Discard after 30 days if stored at room temperature
Discard 30 days after storing at room temperature. Discard after:
(Month) (Day) (Year)
(to be completed by dispensing pharmacist)
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Product of USA
07-01-00-0614
For Intravenous Infusion Only
Micafungin
in 0.9% Sodium Chloride Injection
150 mg per 150 mL (1 mg / mL)
For Intravenous Infusion Only
Micafungin
in 0.9% Sodium Chloride Injection
150 mg per 150 mL (1 mg / mL)
NDC 0338-9055-01
Code 2G3433
Micafungin
in 0.9% Sodium Chloride Injection
150 mg / 150 mL (1 mg / 1 mL)
150 mg
TOTAL
Rx Only
For Intravenous Infusion Only
1 Single-Dose GALAXY Container
Discard unused portion
USE CARTON TO PROECT CONTENTS
FROM LIGHT UNTIL ADMINISTRATION
Baxter Logo









