NDC Code(s) : 0228-3062-11, 0228-3059-11, 0228-3063-11, 0228-3060-11, 0228-3064-11, 0228-3061-11
Packager : Actavis Pharma, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfateDextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfateDextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfateDextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfateDextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfateDextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfateDextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, amphetamine sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Actavis Pharma, Inc.(119723554) |
PRINCIPAL DISPLAY PANEL
NDC 0228-3062-11
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, Amphetamine Sulfate Extended-Release Capsules, CII
(Mixed Salts of a Single Entity Amphetamine Product)
5 mg*
Rx only
100 Capsules

PRINCIPAL DISPLAY PANEL
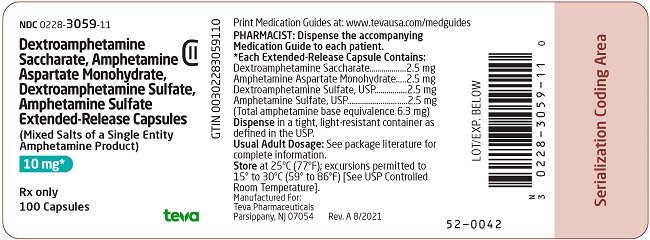
NDC 0228-3059-11
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, Amphetamine Sulfate Extended-Release Capsules, CII
(Mixed Salts of a Single Entity Amphetamine Product)
10 mg*
Rx only
100 Capsules
PRINCIPAL DISPLAY PANEL
NDC 0228-3063-11
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, Amphetamine Sulfate Extended-Release Capsules, CII
(Mixed Salts of a Single Entity Amphetamine Product)
15 mg*
Rx only
100 Capsules

PRINCIPAL DISPLAY PANEL
NDC 0228-3060-11
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, Amphetamine Sulfate Extended-Release Capsules, CII
(Mixed Salts of a Single Entity Amphetamine Product)
20 mg*
Rx only
100 Capsules

PRINCIPAL DISPLAY PANEL
NDC 0228-3064-11
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, Amphetamine Sulfate Extended-Release Capsules, CII
(Mixed Salts of a Single Entity Amphetamine Product)
25 mg*
Rx only
100 Capsules

PRINCIPAL DISPLAY PANEL
NDC 0228-3061-11
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, Amphetamine Sulfate Extended-Release Capsules, CII
(Mixed Salts of a Single Entity Amphetamine Product)
30 mg*
Rx only
100 Capsules










