NDC Code(s) : 0173-0869-10, 0173-0869-06, 0173-0869-61
Packager : GlaxoSmithKline LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Anoro Elliptaumeclidinium bromide and vilanterol trifenatate POWDER | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - GlaxoSmithKline LLC(167380711) |
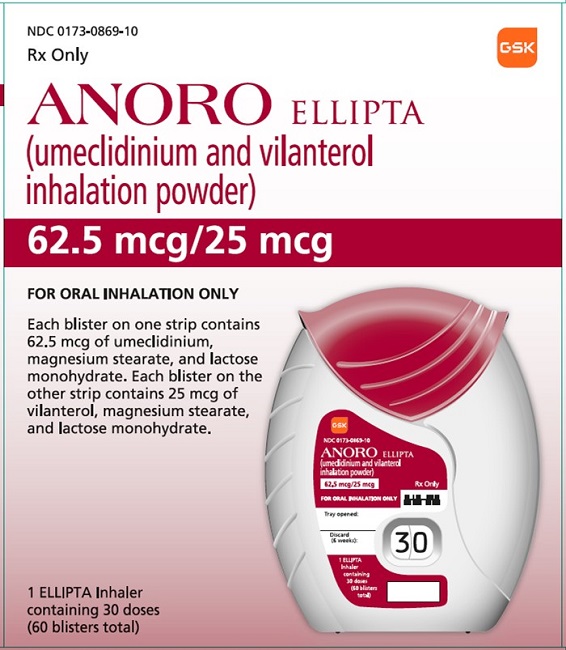
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 0173-0869-10
ANORO ELLIPTA
(umeclidinium and vilanterol inhalation powder)
62.5 mcg/25 mcg
Rx Only
FOR ORAL INHALATION ONLY
Each blister on one strip contains 62.5 mcg of umeclidinium, magnesium stearate, and lactose monohydrate. Each blister on the other strip contains 25 mcg of vilanterol, magnesium stearate, and lactose monohydrate.
1 ELLIPTA Inhaler containing 30 doses (60 blisters total)
GSK
©2023 GSK group of companies or its licensor.
- 62000000084535 Rev.1/23
-