NDC Code(s) : 0169-3687-12, 0169-3687-92, 0169-6438-10, 0169-6438-90, 0169-6438-97, 0169-6438-98, 0169-6432-55, 0169-6432-10
Packager : Novo Nordisk
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Levemirinsulin detemir INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Levemirinsulin detemir INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Levemirinsulin detemir INJECTION, SOLUTION | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| LABELER - Novo Nordisk(622920320) |
PRINCIPAL DISPLAY PANEL
Levemir®
(insulin detemir) injection
NDC 0169-3687-12
List 368712
100 units/mL (U-100)
For subcutaneous use only
Rx Only
Use only with a U-100 syringe.
10 mL Multiple-dose Vial
novo nordisk®

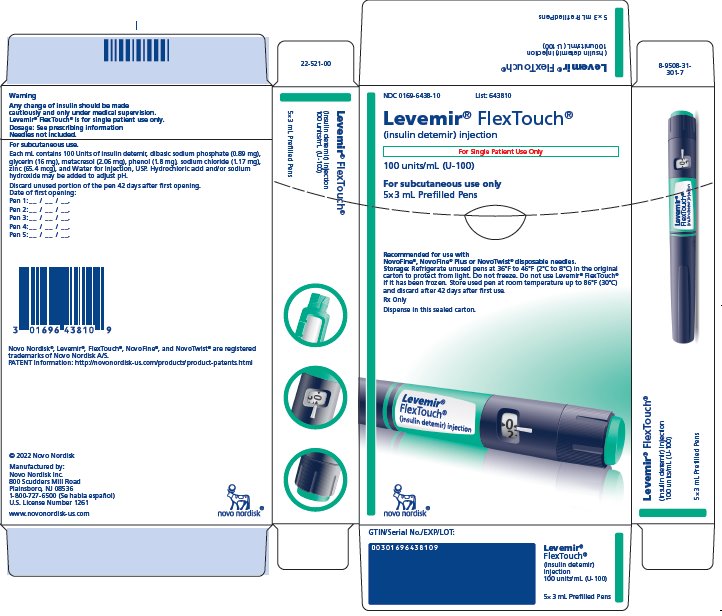
PRINCIPAL DISPLAY PANEL
NDC 0169-6438-10 List 643810
Levemir® FlexTouch®
(insulin detemir) injection
For Single Patient Use Only
100 units/mL (U-100)
For subcutaneous use only
5x3 mL Prefilled Pens
Recommended for use with
NovoFine®, NovoFine® Plus or NovoTwist® disposable needles.
Storage: Refrigerate unused pens at 36°F to 46°F (2°C to 8°C)
in the original carton to protect from light.
Do not freeze. Do not use Levemir® FlexTouch®
if it has been frozen. Store used pen at room temperature
up to 86°F (30°C) and discard after 42 days after first use.
Rx Only
Dispense in this sealed carton.
novo nordisk®
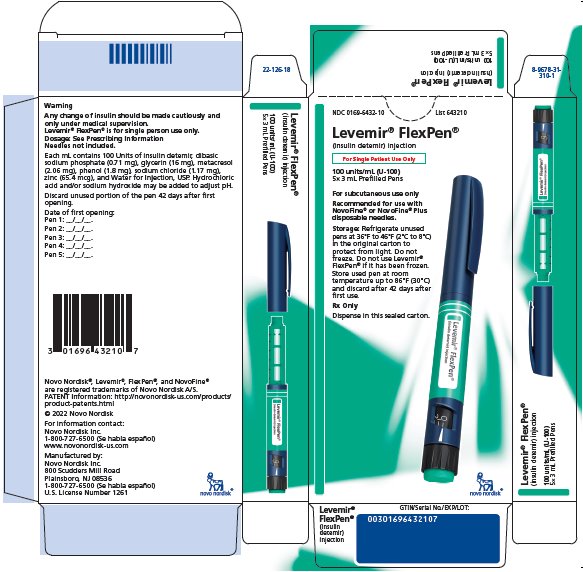
PRINCIPAL DISPLAY PANEL
NDC 0169-6432-10 List 643210
Levemir® FlexPen®
(insulin detemir) injection
For Single Patient Use Only
100 units/mL (U-100)
5 x 3 mL Prefilled Pens
For subcutaneous use only
Recommended for use with NovoFine® or NovoFine® Plus disposable needles.
Storage: Refrigerate unused
pens at 36°F to 46°F (2°C to 8°C)
in the original carton to protect from light.
Do not freeze. Do not use Levemir® FlexPen®
if it has been frozen. Store used pen at room temperature
up to 86°F (30°C) and discard after 42 days after first use.
Rx Only
Dispense in this sealed carton.
novo nordisk®