NDC Code(s) : 0116-2001-16, 0116-2001-04, 0116-2001-15, 0116-2001-05, 0116-2001-06, 0116-2001-07
Packager : Xttrium Laboratories, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Chlorhexidine GluconateChlorhexidine Gluconate RINSE | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Xttrium Laboratories, Inc.(007470579) |
| REGISTRANT - Xttrium Laboratories, Inc.(007470579) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Xttrium Laboratories, Inc. | 007470579 | manufacture(0116-2001) | |
PRINCIPAL DISPLAY PANEL
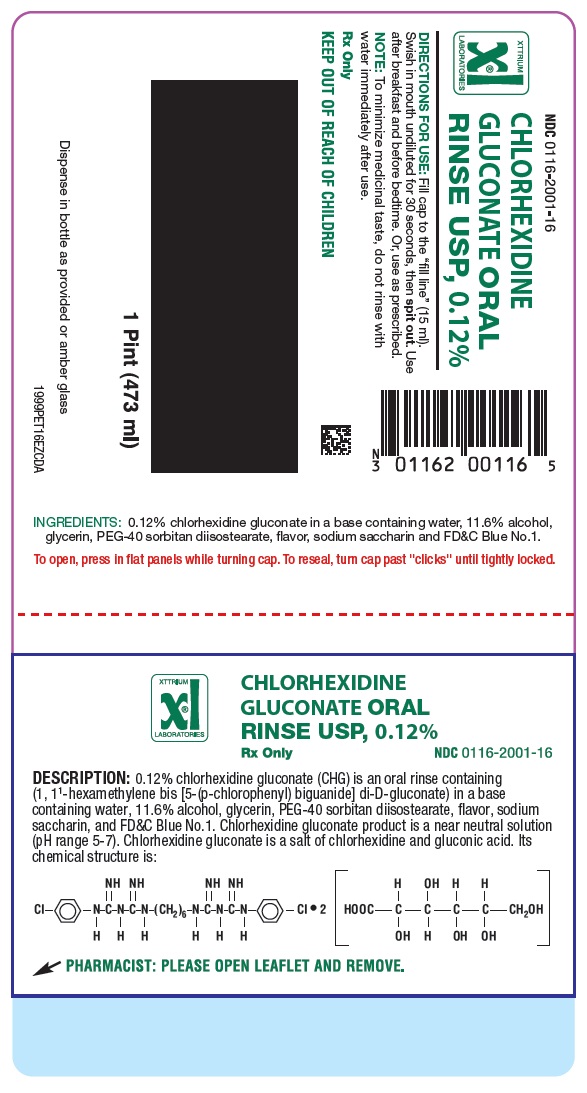
NDC 0116-2001-16
CHLORHEXIDINE GLUCONATE 0.12% ORAL RINSE, USP
Direction for Use: Fill cap to the "fill line" (15ml). Swish in mouth undiluted for 30 seconds, then spit out. Use after breakfast and before bedtime. Or, use as prescribed.
Note: to minimize medicinal taste, do not rinse with water immediately after use.
Rx Only
KEEP OUT OF REACH OF CHILDREN
I PINT (473 ml)
INGREDIENTS: 0.12% chlorhexidine gluconate in a base containing water, 11.6% alcohol, glycerin, PEG-40 sorbitan diisostearate, flavor, sodium saccharin and FD&C Blue No.1.
To open, press in flat pannels while turning cap. To reseal, turn cap past "clicks" until tightly locked.
WHAT TO EXPECT WHEN USING CHLORHEXIDINE GLUCONATE ORAL RINSE
Your dentist has prescribed chlorhexidine gluconate oral rinse to treat your gingivitis, to help reduce the redness and swelling of your gums, and also to help you control any gum bleeding. Use chlorhexidine gluconate oral rinse regularly, as directed by your dentist, in addition to daily brushing. Spit out after use, chlorhexidine gluconate oral rinse should not be swallowed.
If you develop allergic symptoms such as skin rash, itch, generalized swelling, breathing difficulties, light headedness, rapid heart rate, upset stomach or diarrhea, seek medical attention immediately. Chlorhexidine gluconate oral rinse should not be used by persons who have a sensitivity to it or its components.
Chlorhexidine gluconate oral rinse may cause some tooth discoloration, or increase in tartar (calculus) formation, particularly in areas where stain and tartar usually form. It is important to see your dentist for removal of any stain or tartar at least every six months or more frequently if your dentist advises.
- Both stain and tartar can be removed by your dentist or hygienist. Chlorhexidine gluconate oral rinse may cause permanent discoloration of some front-tooth fillings.
- To minimize discoloration, you should brush and floss daily, emphasizing areas which begin to discolor.
- Chlorhexidine gluconate oral rinse may taste bitter to some patients and can affect how food and beverages taste. This will become less noticeable in most cases with continued use of chlorhexidine gluconate oral rinse.
- To avoid taste interference, rinse with chlorhexidine gluconate oral rinse after meals. Do not rinse with water or other mouthwashes immediately after rinsing with chlorhexidine gluconate oral rinse.
If you have any questions or comments about Chlorhexidine gluconate oral rinse, contact your dentist, pharmacist, or Xttrium Laboratories, Inc. toll free at 1-800-587-3721.
Call your health care provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
STORE at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP controlled Room Temperature].



PRINCIPAL DISPLAY PANEL
NDC 0116-2001-04
CHLORHEXIDINE GLUCONATE 0.12% ORAL RINSE, USP
Direction for Use: Fill cap to the "fill line" (15ml). Swish in mouth undiluted for 30 seconds, then spit out. Use after breakfast and before bedtime. Or, use as prescribed.
Note: to minimize medicinal taste, do not rinse with water immediately after use.
Rx Only
KEEP OUT OF REACH OF CHILDREN
4 oz. (118 mL)
INGREDIENTS: 0.12% chlorhexidine gluconate in a base containing water, 11.6% alcohol, glycerin, PEG-40 sorbitan diisostearate, flavor, sodium saccharin and FD&C Blue No.1.
To open, press in flat pannels while turning cap. To reseal, turn cap past "clicks" until tightly locked.
WHAT TO EXPECT WHEN USING CHLORHEXIDINE GLUCONATE ORAL RINSE
Your dentist has prescribed chlorhexidine gluconate oral rinse to treat your gingivitis, to help reduce the redness and swelling of your gums, and also to help you control any gum bleeding. Use chlorhexidine gluconate oral rinse regularly, as directed by your dentist, in addition to daily brushing. Spit out after use, chlorhexidine gluconate oral rinse should not be swallowed.
If you develop allergic symptoms such as skin rash, itch, generalized swelling, breathing difficulties, light headedness, rapid heart rate, upset stomach or diarrhea, seek medical attention immediately. Chlorhexidine gluconate oral rinse should not be used by persons who have a sensitivity to it or its components.
Chlorhexidine gluconate oral rinse may cause some tooth discoloration, or increase in tartar (calculus) formation, particularly in areas where stain and tartar usually form. It is important to see your dentist for removal of any stain or tartar at least every six months or more frequently if your dentist advises.
Both stain and tartar can be removed by your dentist or hygienist. Chlorhexidine gluconate oral rinse may cause permanent discoloration of some front-tooth fillings.
To minimize discoloration, you should brush and floss daily, emphasizing areas which begin to discolor.
Chlorhexidine gluconate oral rinse may taste bitter to some patients and can affect how food and beverages taste. This will become less noticeable in most cases with continued use of chlorhexidine gluconate oral rinse.
To avoid taste interference, rinse with chlorhexidine gluconate oral rinse after meals. Do not rinse with water or other mouthwashes immediately after rinsing with chlorhexidine gluconate oral rinse.
If you have any questions or comments about Chlorhexidine gluconate oral rinse, contact your dentist, pharmacist, or Xttrium Laboratories, Inc. toll free at 1-800-587-3721.
Call your health care provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
STORE at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP controlled Room Temperature].



PRINCIPAL DISPLAY PANEL
NDC 0116-2001-15
CHLORHEXIDINE GLUCONATE 0.12% ORAL RINSE, USP
Direction for Use: Swish in mouth undiluted for 30 seconds, then spit out. Use after breakfast and before bedtime. Or, use as prescribed. NOTE: To minimize medicinal taste, do not rinse with water
immediately after use.
Note: to minimize medicinal taste, do not rinse with water immediately after use.
Rx Only
KEEP OUT OF REACH OF CHILDREN
1 oz. (15 mL)
INGREDIENTS: 0.12% chlorhexidine gluconate in a base containing water, 11.6% alcohol, glycerin, PEG-40 sorbitan diisostearate, flavor, sodium saccharin and FD&C Blue No.1.
To open, press in flat pannels while turning cap. To reseal, turn cap past "clicks" until tightly locked.
WHAT TO EXPECT WHEN USING CHLORHEXIDINE GLUCONATE ORAL RINSE
Your dentist has prescribed chlorhexidine gluconate oral rinse to treat your gingivitis, to help reduce the redness and swelling of your gums, and also to help you control any gum bleeding. Use chlorhexidine gluconate oral rinse regularly, as directed by your dentist, in addition to daily brushing. Spit out after use, chlorhexidine gluconate oral rinse should not be swallowed.
If you develop allergic symptoms such as skin rash, itch, generalized swelling, breathing difficulties, light headedness, rapid heart rate, upset stomach or diarrhea, seek medical attention immediately. Chlorhexidine gluconate oral rinse should not be used by persons who have a sensitivity to it or its components.
Chlorhexidine gluconate oral rinse may cause some tooth discoloration, or increase in tartar (calculus) formation, particularly in areas where stain and tartar usually form. It is important to see your dentist for removal of any stain or tartar at least every six months or more frequently if your dentist advises.
Both stain and tartar can be removed by your dentist or hygienist. Chlorhexidine gluconate oral rinse may cause permanent discoloration of some front-tooth fillings.
To minimize discoloration, you should brush and floss daily, emphasizing areas which begin to discolor.
Chlorhexidine gluconate oral rinse may taste bitter to some patients and can affect how food and beverages taste. This will become less noticeable in most cases with continued use of chlorhexidine gluconate oral rinse.
To avoid taste interference, rinse with chlorhexidine gluconate oral rinse after meals. Do not rinse with water or other mouthwashes immediately after rinsing with chlorhexidine gluconate oral rinse.
If you have any questions or comments about Chlorhexidine gluconate oral rinse, contact your dentist, pharmacist, or Xttrium Laboratories, Inc. toll free at 1-800-587-3721.
Call your health care provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
STORE at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP controlled Room Temperature].





PRINCIPAL DISPLAY PANEL
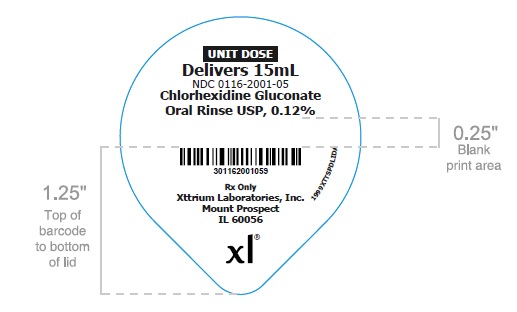
UNIT DOSE
Delivers 15mL
NDC 0116-2001-05
Chlorhexidine Gluconate
Oral Rinse USP, 0.12%
Rx Only
Xttrium Laboratories, Inc.
Mount Prospect, IL 60056
1999XTTSPDLIDA
1999CHGUDCINSTA













