NDC Code(s) : 0115-1695-30, 0115-1695-49, 0115-1694-30, 0115-1694-49
Packager : Amneal Pharmaceuticals of New York LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| epinephrineepinephrine INJECTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| epinephrineepinephrine INJECTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Amneal Pharmaceuticals of New York LLC(123797875) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Boehringer Ingelheim Pharma GmbH and Co. KG | 551147440 | api manufacture(0115-1695, 0115-1694) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Hospira, Inc. | 030606222 | analysis(0115-1695, 0115-1694), manufacture(0115-1695, 0115-1694) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| PPD Development, L.P. | 838082055 | analysis(0115-1695, 0115-1694) | |
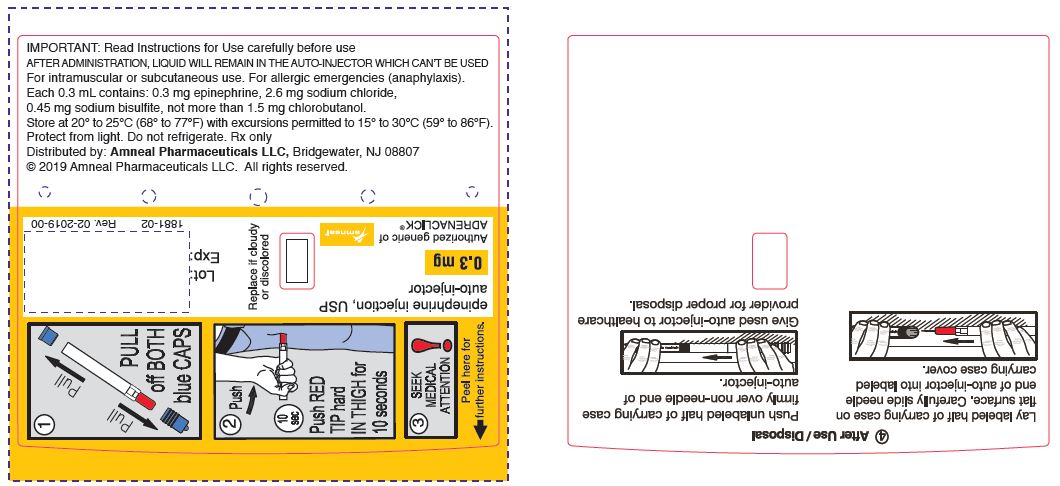
PRINCIPAL DISPLAY PANEL
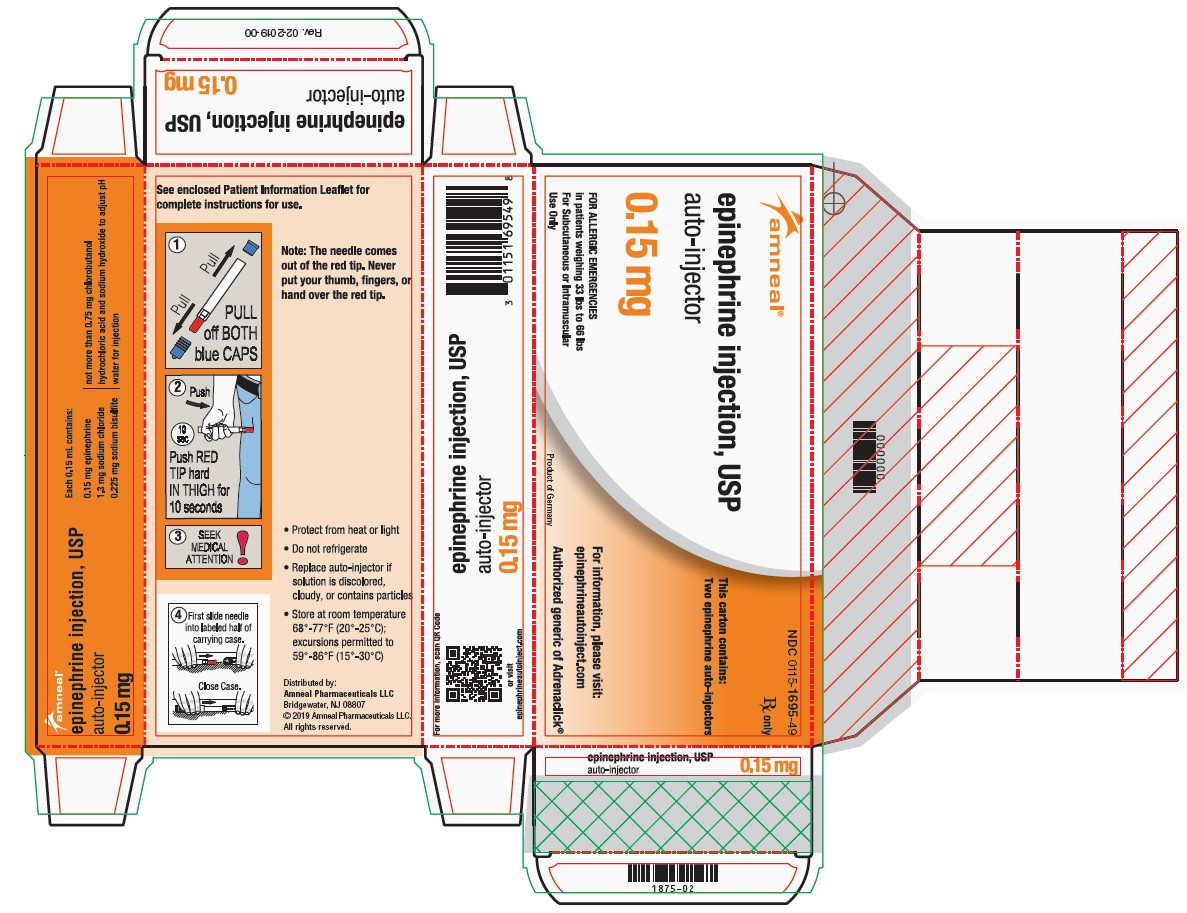
PRINCIPAL DISPLAY PANEL
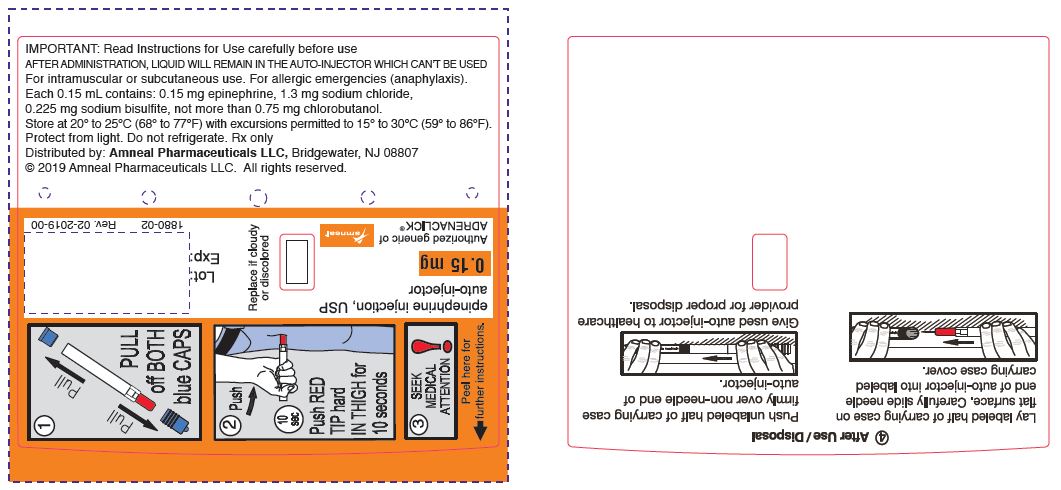
PRINCIPAL DISPLAY PANEL
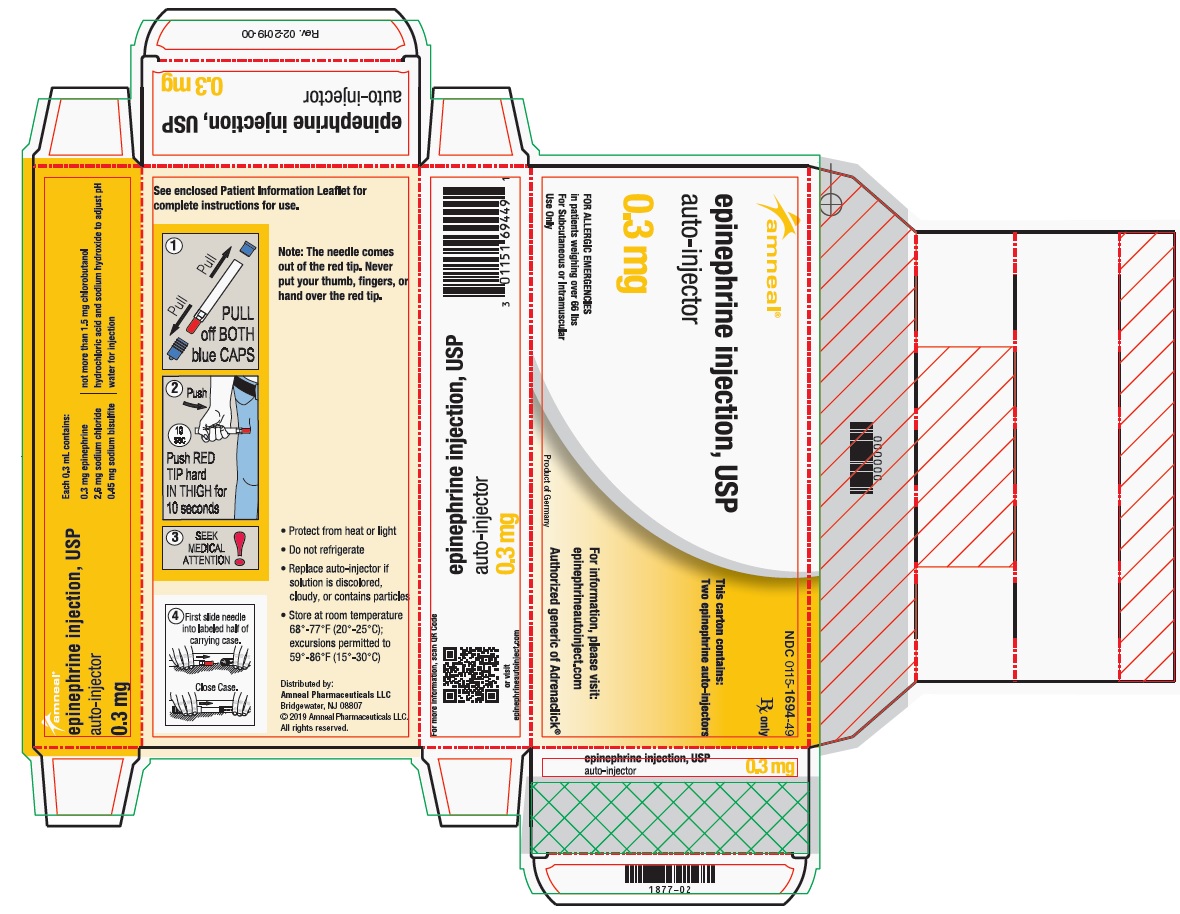
PRINCIPAL DISPLAY PANEL