NDC Code(s) : 0115-1486-01, 0115-1487-01, 0115-1488-01, 0115-1489-01, 0115-1490-01, 0115-1491-01
Packager : Amneal Pharmaceuticals of New York LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine SulfateDextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate CAPSULE | ||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine SulfateDextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate CAPSULE | ||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine SulfateDextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate CAPSULE | ||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine SulfateDextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate CAPSULE | ||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine SulfateDextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate CAPSULE | ||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine SulfateDextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate CAPSULE | ||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Amneal Pharmaceuticals of New York LLC(123797875) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Amneal Pharmaceuticals of New York, LLC | 123797875 | label(0115-1486, 0115-1487, 0115-1488, 0115-1489, 0115-1490, 0115-1491), pack(0115-1486, 0115-1487, 0115-1488, 0115-1489, 0115-1490, 0115-1491) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Catalent Pharma Solutions, LLC | 829672745 | analysis(0115-1486, 0115-1487, 0115-1488, 0115-1489, 0115-1490, 0115-1491), manufacture(0115-1486, 0115-1487, 0115-1488, 0115-1489, 0115-1490, 0115-1491) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| CorePharma, LLC | 031192276 | label(0115-1486, 0115-1487, 0115-1488, 0115-1489, 0115-1490, 0115-1491), pack(0115-1486, 0115-1487, 0115-1488, 0115-1489, 0115-1490, 0115-1490, 0115-1491) | |
PRINCIPAL DISPLAY PANEL
NDC 0115-1486-01
CII
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine Sulfate (Mixed Salts of a Single-Entity Amphetamine Product) Extended-Release Capsules 5 mg
Rx only
100 Capsules

PRINCIPAL DISPLAY PANEL
NDC 0115-1487-01
CII
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine Sulfate (Mixed Salts of a Single-Entity Amphetamine Product) Extended-Release Capsules 10 mg
Rx only
100 Capsules

PRINCIPAL DISPLAY PANEL
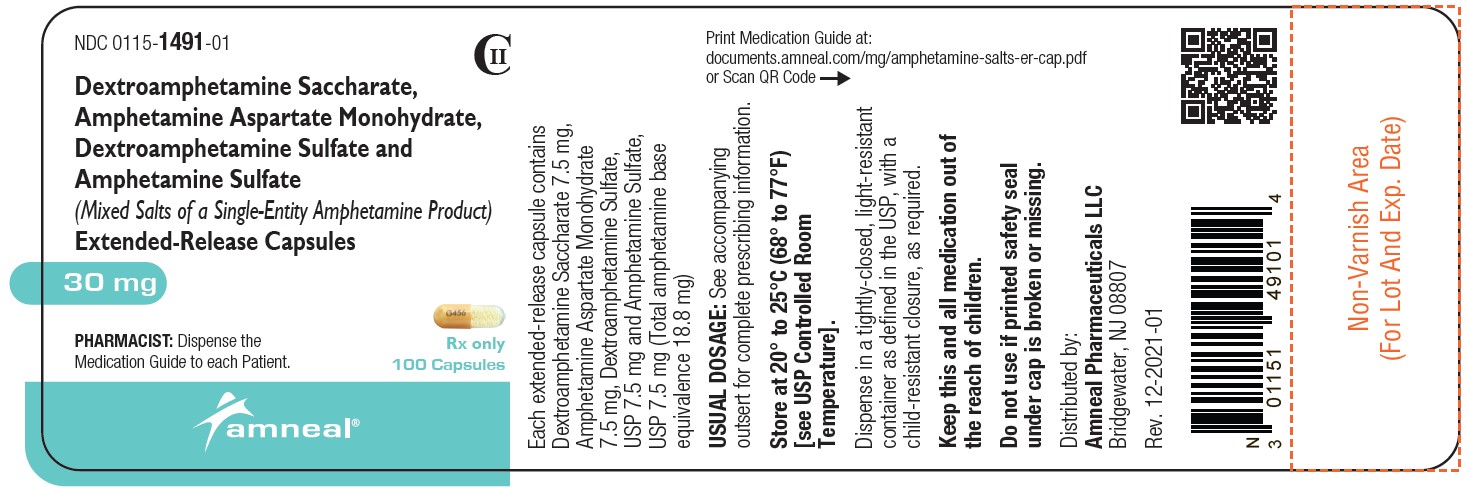
NDC 0115-1488-01
CII
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine Sulfate (Mixed Salts of a Single-Entity Amphetamine Product) Extended-Release Capsules 15 mg
Rx only
100 Capsules
PRINCIPAL DISPLAY PANEL
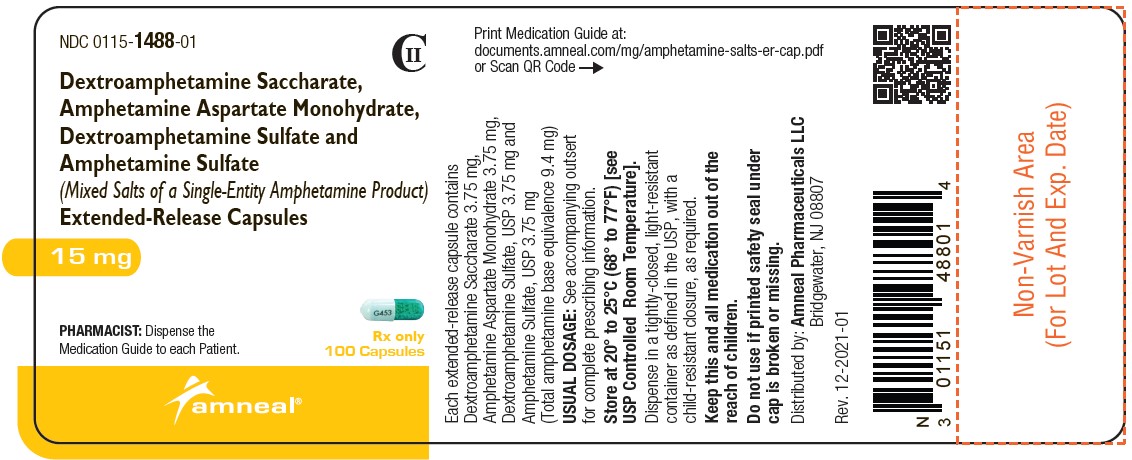
NDC 0115-1489-01
CII
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine Sulfate (Mixed Salts of a Single-Entity Amphetamine Product) Extended-Release Capsules 20 mg
Rx only
100 Capsules
PRINCIPAL DISPLAY PANEL
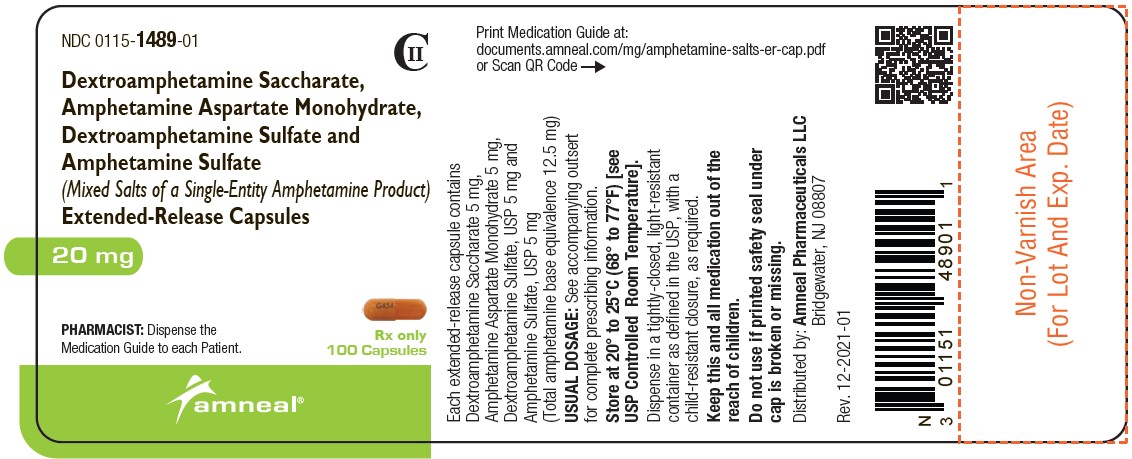
NDC 0115-1490-01
CII
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine Sulfate (Mixed Salts of a Single-Entity Amphetamine Product) Extended-Release Capsules 25 mg
Rx only
100 Capsules
PRINCIPAL DISPLAY PANEL
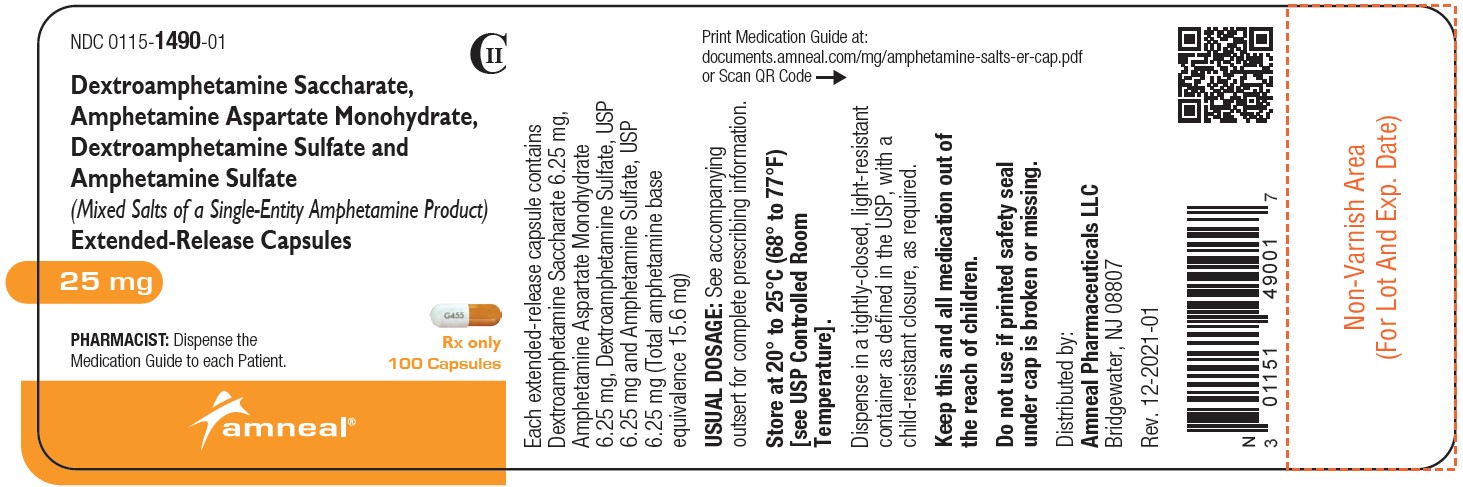
NDC 0115-1491-01
CII
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine Sulfate (Mixed Salts of a Single-Entity Amphetamine Product) Extended-Release Capsules 30 mg
Rx only
100 Capsules