NDC Code(s) : 0093-2263-01, 0093-2264-01, 0093-4160-76, 0093-4160-78, 0093-4160-73, 0093-4155-79, 0093-4155-73, 0093-4155-80, 0093-4161-76, 0093-4161-78, 0093-4161-73, 0093-2267-01, 0093-2268-01, 0093-3107-01, 0093-3107-05, 0093-3109-53, 0093-3109-06, 0093-3109-01, 0093-3109-05
Packager : Teva Pharmaceuticals USA, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AmoxicillinAmoxicillin TABLET, FILM COATED | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| AmoxicillinAmoxicillin TABLET, FILM COATED | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| AmoxicillinAmoxicillin POWDER, FOR SUSPENSION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| AmoxicillinAmoxicillin POWDER, FOR SUSPENSION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| AmoxicillinAmoxicillin POWDER, FOR SUSPENSION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| AmoxicillinAmoxicillin TABLET, CHEWABLE | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| AmoxicillinAmoxicillin TABLET, CHEWABLE | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| AmoxicillinAmoxicillin CAPSULE | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| AmoxicillinAmoxicillin CAPSULE | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| LABELER - Teva Pharmaceuticals USA, Inc.(001627975) |
PRINCIPAL DISPLAY PANEL
NDC 0093-2263-01
Amoxicillin Tablets, USP
500 mg
AMOXICILLIN USP, 500 mg as the trihydrate
Rx only
100 Tablets

PRINCIPAL DISPLAY PANEL
NDC 0093-2264-01
Amoxicillin Tablets, USP
875 mg
AMOXICILLIN USP, 875 mg as the trihydrate
Rx only
100 Tablets

PRINCIPAL DISPLAY PANEL
NDC 0093-4160-73
Amoxicillin for Oral Suspension, USP
200 mg per 5 mL
When reconstituted, each 5 mL contains: AMOXICILLIN USP, 200 mg as the trihydrate
Rx only
100 mL (when reconstituted)

PRINCIPAL DISPLAY PANEL
NDC 0093-4155-73
Amoxicillin for Oral Suspension, USP
250 mg per 5 mL
When reconstituted, each 5 mL contains: AMOXICILLIN USP, 250 mg as the trihydrate
Rx only
100 mL (when reconstituted)

PRINCIPAL DISPLAY PANEL
NDC 0093-4161-73
Amoxicillin for Oral Suspension, USP
400 mg per 5 mL
When reconstituted, each 5 mL contains: AMOXICILLIN USP, 400 mg as the trihydrate
Rx only
100 mL (when reconstituted)

PRINCIPAL DISPLAY PANEL
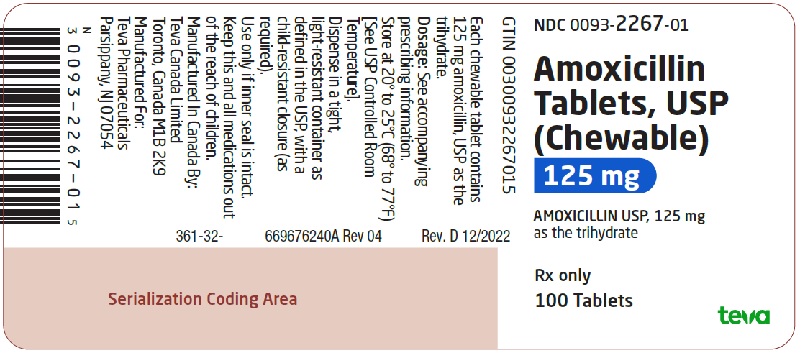
NDC 0093-2267-01
Amoxicillin Tablets, USP
(Chewable)
125 mg
AMOXICILLIN USP, 125 mg as the trihydrate
Rx only
100 Tablets
PRINCIPAL DISPLAY PANEL
NDC 0093-2268-01
Amoxicillin Tablets, USP
(Chewable)
250 mg
AMOXICILLIN USP, 250 mg as the trihydrate
Rx only
100 Tablets

PRINCIPAL DISPLAY PANEL
NDC 0093-3107-05
Amoxicillin Capsules, USP
250 mg
Rx only
500 Capsules

PRINCIPAL DISPLAY PANEL
NDC 0093-3109-05
Amoxicillin Capsules, USP
500 mg
Rx only
500 Capsules