NDC Code(s) : 0085-3305-30, 0085-3310-30, 0085-3310-35, 0085-3315-30, 0085-3315-35, 0085-3320-30, 0085-3320-35, 0085-3330-30, 0085-0819-30, 0085-0819-35
Packager : Merck Sharp & Dohme Corp.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| NITRO-DURnitroglycerin PATCH | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NITRO-DURnitroglycerin PATCH | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NITRO-DURnitroglycerin PATCH | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NITRO-DURnitroglycerin PATCH | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NITRO-DURnitroglycerin PATCH | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NITRO-DURnitroglycerin PATCH | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
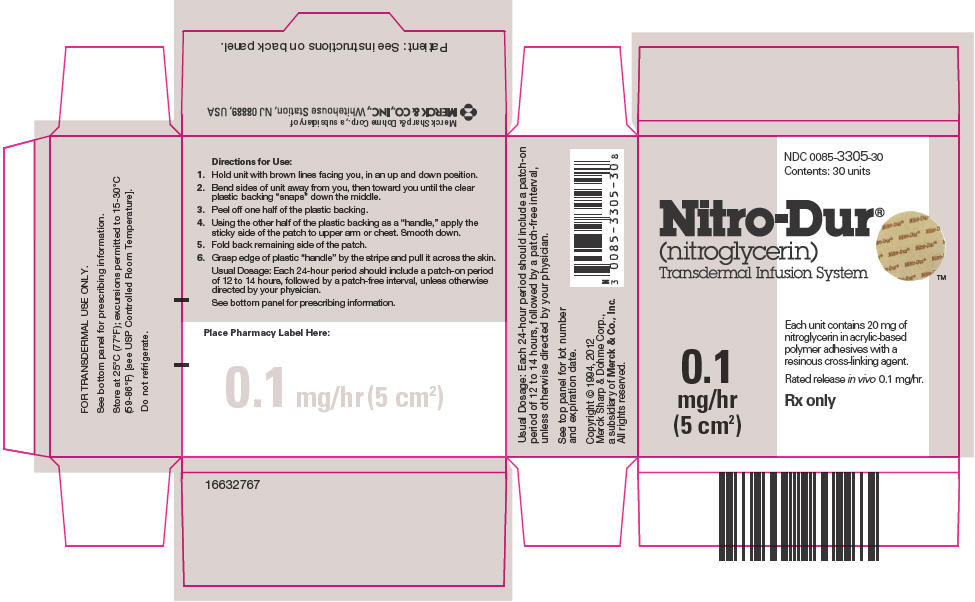
PRINCIPAL DISPLAY PANEL
NDC 0085-3305-30
Contents: 30 units
Nitro-Dur
®
(nitroglycerin)
Transdermal Infusion System
0.1
mg/hr
(5 cm2)
Each unit contains 20 mg of
nitroglycerin in acrylic-based
polymer adhesives with a
resinous cross-linking agent.
Rated release in vivo 0.1 mg/hr.
Rx only
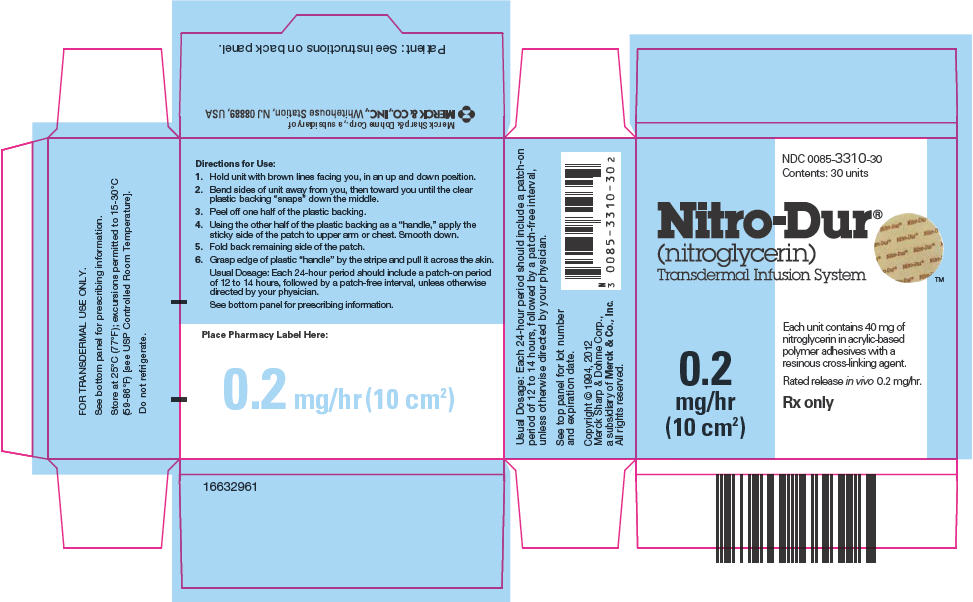
PRINCIPAL DISPLAY PANEL
NDC 0085-3310-30
Contents: 30 units
Nitro-Dur
®
(nitroglycerin)
Transdermal Infusion System
0.2
mg/hr
(10 cm2)
Each unit contains 40 mg of
nitroglycerin in acrylic-based
polymer adhesives with a
resinous cross-linking agent.
Rated release in vivo 0.2 mg/hr.
Rx only
PRINCIPAL DISPLAY PANEL
NDC 0085-3315-30
Contents: 30 units
Nitro-Dur
®
(nitroglycerin)
Transdermal Infusion System
0.3
mg/hr
(15 cm2)
Each unit contains 60 mg of
nitroglycerin in acrylic-based
polymer adhesives with a
resinous cross-linking agent.
Rated release in vivo 0.3 mg/hr.
Rx only

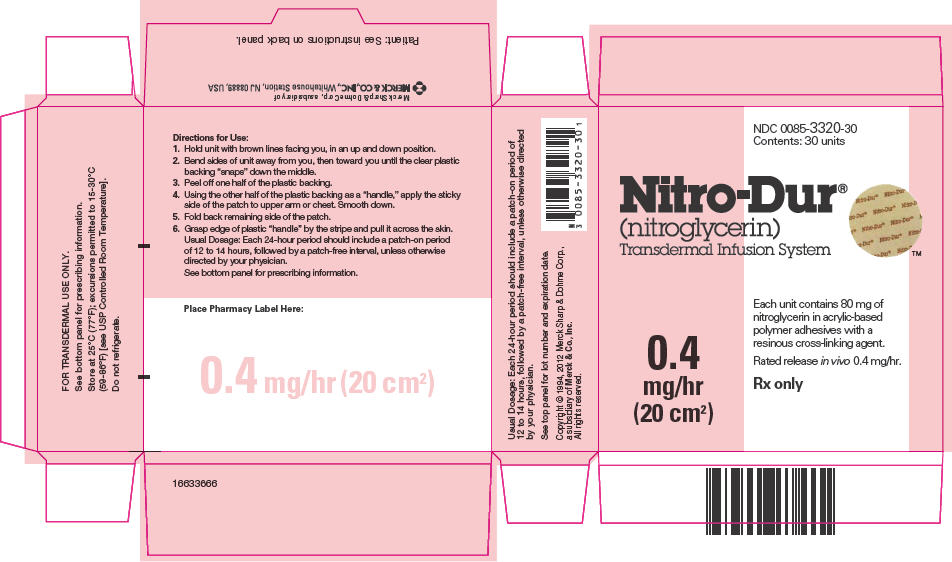
PRINCIPAL DISPLAY PANEL
NDC 0085-3320-30
Contents: 30 units
Nitro-Dur
®
(nitroglycerin)
Transdermal Infusion System
0.4
mg/hr
(20 cm2)
Each unit contains 80 mg of
nitroglycerin in acrylic-based
polymer adhesives with a
resinous cross-linking agent.
Rated release in vivo 0.4 mg/hr.
Rx only
PRINCIPAL DISPLAY PANEL
NDC 0085-3330-30
Contents: 30 units
Nitro-Dur
®
(nitroglycerin)
Transdermal Infusion System
0.6
mg/hr
(30 cm2)
Each unit contains 120 mg of
nitroglycerin in acrylic-based
polymer adhesives with a
resinous cross-linking agent.
Rated release in vivo 0.6 mg/hr.
Rx only

PRINCIPAL DISPLAY PANEL
NDC 0085-0819-30
Contents: 30 units
Nitro-Dur
®
(nitroglycerin)
Transdermal Infusion System
0.8
mg/hr
(40 cm2)
Each unit contains 160 mg of
nitroglycerin in acrylic-based
polymer adhesives with a
resinous cross-linking agent.
Rated release in vivo 0.8 mg/hr.
Rx only










