NDC Code(s) : 0069-2150-30, 0069-2170-30, 0069-2190-30, 0069-2260-30, 0069-2160-30, 0069-2180-30, 0069-2250-30, 0069-2270-30, 0069-6180-30, 0069-6323-30, 0069-6565-30, 0069-6747-30, 0069-7810-30, 0069-7232-30, 0069-7654-30, 0069-7476-30
Packager : Pfizer Laboratories Div Pfizer Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Caduetamlodipine besylate and atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Caduetamlodipine besylate and atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Caduetamlodipine besylate and atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Caduetamlodipine besylate and atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Caduetamlodipine besylate and atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Caduetamlodipine besylate and atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Caduetamlodipine besylate and atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Caduetamlodipine besylate and atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Caduetamlodipine and atorvastatin TABLET, FILM COATED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Caduetamlodipine and atorvastatin TABLET, FILM COATED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Caduetamlodipine and atorvastatin TABLET, FILM COATED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Caduetamlodipine and atorvastatin TABLET, FILM COATED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Caduetamlodipine and atorvastatin TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Caduetamlodipine and atorvastatin TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Caduetamlodipine and atorvastatin TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Caduetamlodipine and atorvastatin TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Pfizer Laboratories Div Pfizer Inc(134489525) |
| REGISTRANT - Pfizer Inc(113480771) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Viatris Pharmaceuticals LLC | 829084545 | API MANUFACTURE(0069-2150, 0069-2160, 0069-2170, 0069-2180, 0069-2190, 0069-2250, 0069-2260, 0069-2270, 0069-6180, 0069-6323, 0069-6565, 0069-6747, 0069-7232, 0069-7476, 0069-7654, 0069-7810) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pharmacia & Upjohn Company LLC | 618054084 | PACK(0069-2150, 0069-2170, 0069-2190, 0069-2260, 0069-2160, 0069-2180, 0069-2250, 0069-2270, 0069-6180, 0069-6323, 0069-6565, 0069-6747, 0069-7810, 0069-7232, 0069-7654, 0069-7476), LABEL(0069-2150, 0069-2170, 0069-2190, 0069-2260, 0069-2160, 0069-2180, 0069-2250, 0069-2270, 0069-6180, 0069-6323, 0069-6565, 0069-6747, 0069-7810, 0069-7232, 0069-7654, 0069-7476) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Viatris Pharmaceuticals LLC | 829084552 | PACK(0069-2150, 0069-2160, 0069-2170, 0069-2180, 0069-2190, 0069-2250, 0069-2260, 0069-2270, 0069-6180, 0069-6323, 0069-6565, 0069-6747, 0069-7232, 0069-7476, 0069-7654, 0069-7810) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pfizer Ireland Pharmaceuticals | 985052076 | ANALYSIS(0069-2150, 0069-2160, 0069-2170, 0069-2180, 0069-2190, 0069-2250, 0069-2260, 0069-2270, 0069-6180, 0069-6323, 0069-6565, 0069-6747, 0069-7232, 0069-7476, 0069-7654, 0069-7810), API MANUFACTURE(0069-2150, 0069-2160, 0069-2170, 0069-2180, 0069-2190, 0069-2250, 0069-2260, 0069-2270, 0069-6180, 0069-6323, 0069-6565, 0069-6747, 0069-7232, 0069-7476, 0069-7654, 0069-7810) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pfizer Manufacturing Deutschland GmbH | 341970073 | ANALYSIS(0069-2150, 0069-2160, 0069-2170, 0069-2180, 0069-2190, 0069-2250, 0069-2260, 0069-2270, 0069-6180, 0069-6323, 0069-6565, 0069-6747, 0069-7232, 0069-7476, 0069-7654, 0069-7810), MANUFACTURE(0069-2150, 0069-2160, 0069-2170, 0069-2180, 0069-2190, 0069-2250, 0069-2260, 0069-2270, 0069-6180, 0069-6323, 0069-6565, 0069-6747, 0069-7232, 0069-7476, 0069-7654, 0069-7810), PACK(0069-2150, 0069-2160, 0069-2170, 0069-2180, 0069-2190, 0069-2250, 0069-2260, 0069-2270, 0069-6180, 0069-6323, 0069-6565, 0069-6747, 0069-7232, 0069-7476, 0069-7654, 0069-7810), LABEL(0069-2150, 0069-2160, 0069-2170, 0069-2180, 0069-2190, 0069-2250, 0069-2260, 0069-2270, 0069-6180, 0069-6323, 0069-6565, 0069-6747, 0069-7232, 0069-7476, 0069-7654, 0069-7810) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pfizer Inc | 943955690 | ANALYSIS(0069-2150, 0069-2170, 0069-2190, 0069-2260, 0069-2160, 0069-2180, 0069-2250, 0069-2270, 0069-6180, 0069-6323, 0069-6565, 0069-6747, 0069-7810, 0069-7232, 0069-7654, 0069-7476) | |
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 5 mg/10 mg Tablet Bottle Label
Pfizer
NDC 0069-2150-30
Caduet®
(amlodipine besylate/
atorvastatin calcium)
Tablets
5 mg/10 mg*
30 Tablets
Rx only

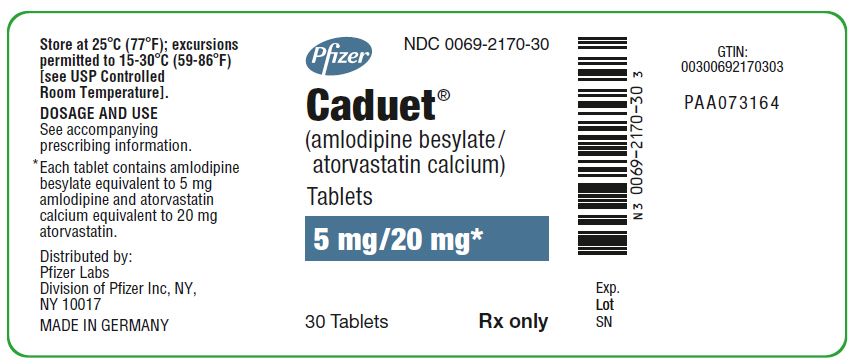
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 5 mg/20 mg Tablet Bottle Label
Pfizer
NDC 0069-2170-30
Caduet®
(amlodipine besylate/
atorvastatin calcium)
Tablets
5 mg/20 mg*
30 Tablets
Rx only
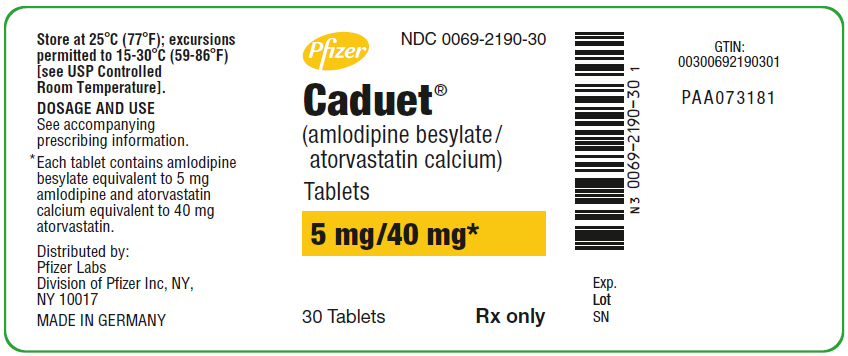
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 5 mg/40 mg Tablet Bottle Label
Pfizer
NDC 0069-2190-30
Caduet®
(amlodipine besylate/
atorvastatin calcium)
Tablets
5 mg/40 mg*
30 Tablets
Rx only
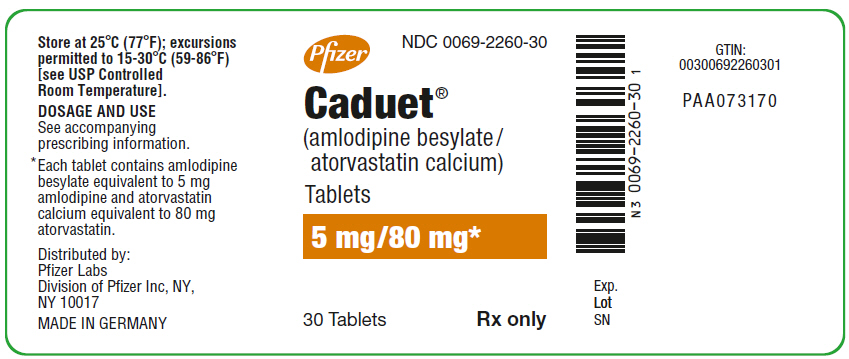
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 5 mg/80 mg Tablet Bottle Label
Pfizer
NDC 0069-2260-30
Caduet®
(amlodipine besylate/
atorvastatin calcium)
Tablets
5 mg/80 mg*
30 Tablets
Rx only
PRINCIPAL DISPLAY PANEL
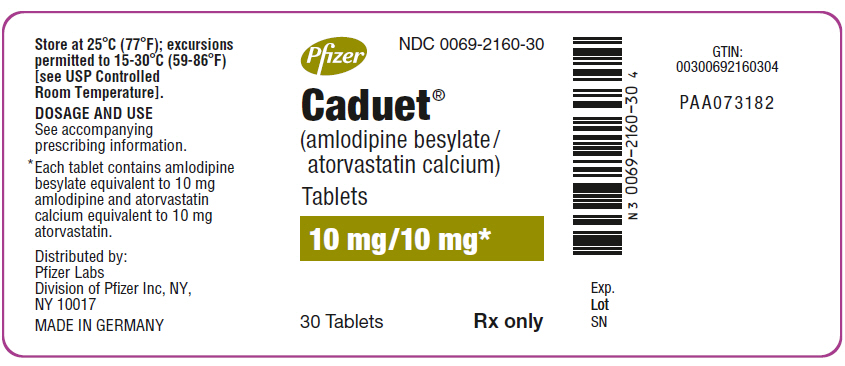
PRINCIPAL DISPLAY PANEL - 10 mg/10 mg Tablet Bottle Label
Pfizer
NDC 0069-2160-30
Caduet®
(amlodipine besylate/
atorvastatin calcium)
Tablets
10 mg/10 mg*
30 Tablets
Rx only
PRINCIPAL DISPLAY PANEL
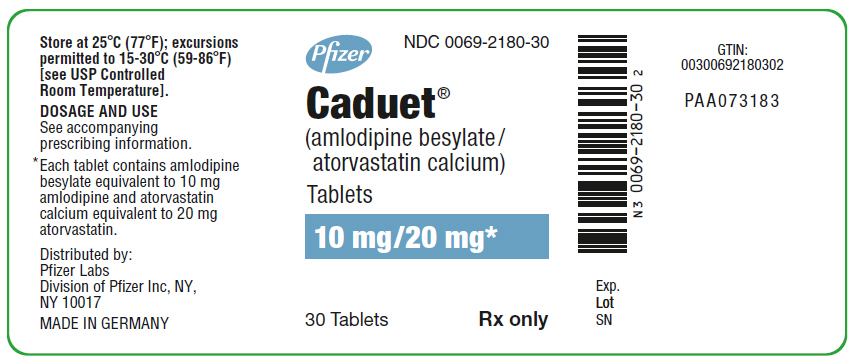
PRINCIPAL DISPLAY PANEL - 10 mg/20 mg Tablet Bottle Label
Pfizer
NDC 0069-2180-30
Caduet®
(amlodipine besylate/
atorvastatin calcium)
Tablets
10 mg/20 mg*
30 Tablets
Rx only
PRINCIPAL DISPLAY PANEL
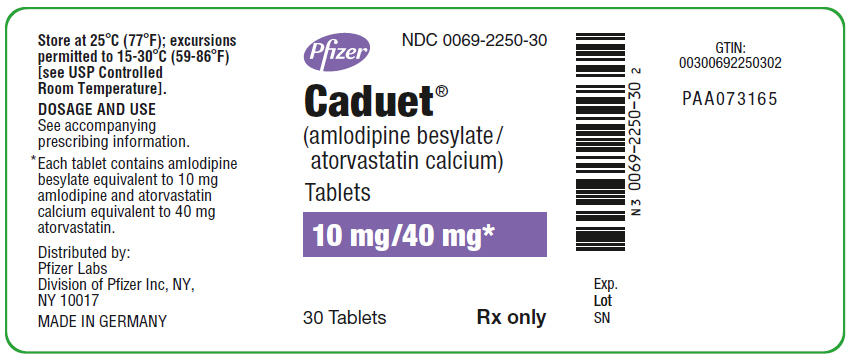
PRINCIPAL DISPLAY PANEL - 10 mg/40 mg Tablet Bottle Label
Pfizer
NDC 0069-2250-30
Caduet®
(amlodipine besylate/
atorvastatin calcium)
Tablets
10 mg/40 mg*
30 Tablets
Rx only
PRINCIPAL DISPLAY PANEL
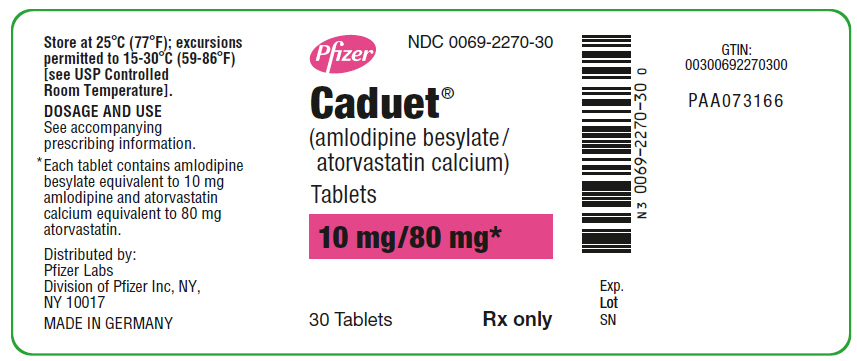
PRINCIPAL DISPLAY PANEL - 10 mg/80 mg Tablet Bottle Label
Pfizer
NDC 0069-2270-30
Caduet®
(amlodipine besylate/
atorvastatin calcium)
Tablets
10 mg/80 mg*
30 Tablets
Rx only
PRINCIPAL DISPLAY PANEL
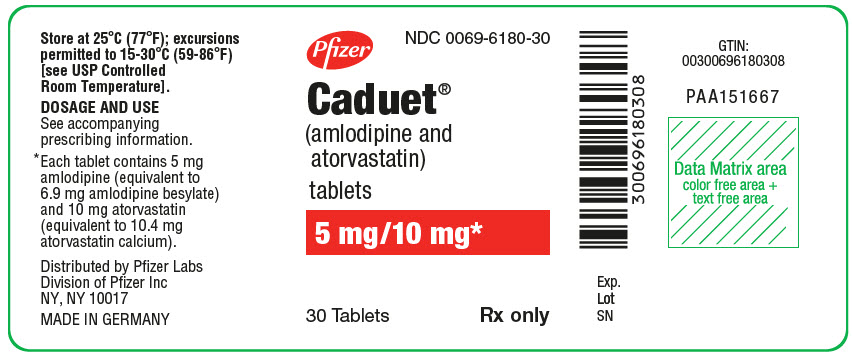
Pfizer
NDC 0069-6180-30
Caduet®
(amlodipine and
atorvastatin)
tablets
5 mg/10 mg*
30 Tablets
Rx only
PRINCIPAL DISPLAY PANEL
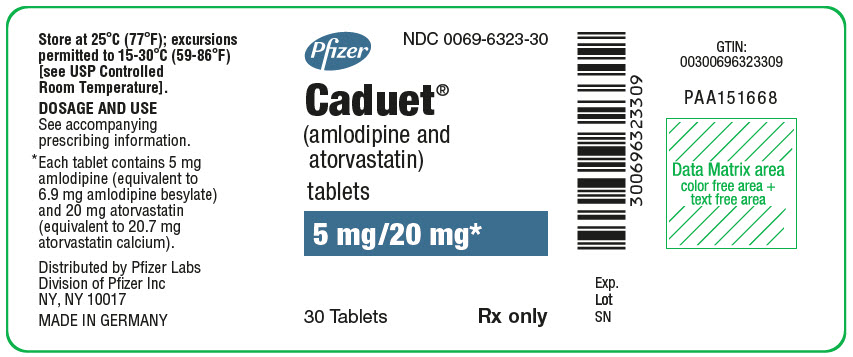
Pfizer
NDC 0069-6323-30
Caduet®
(amlodipine and
atorvastatin)
tablets
5 mg/20 mg*
30 Tablets
Rx only
PRINCIPAL DISPLAY PANEL
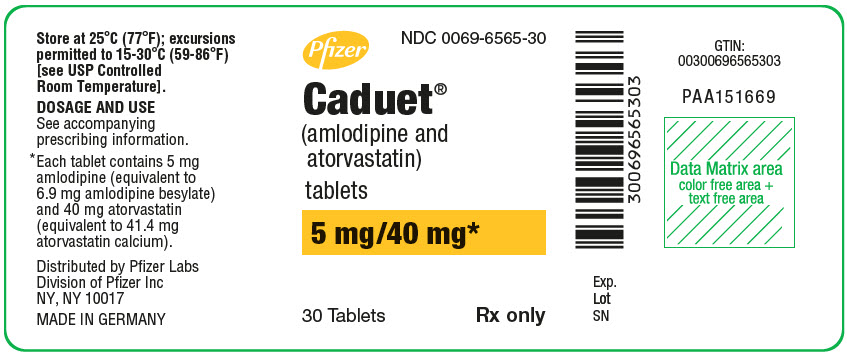
Pfizer
NDC 0069-6565-30
Caduet®
(amlodipine and
atorvastatin)
tablets
5 mg/40 mg*
30 Tablets
Rx only
PRINCIPAL DISPLAY PANEL
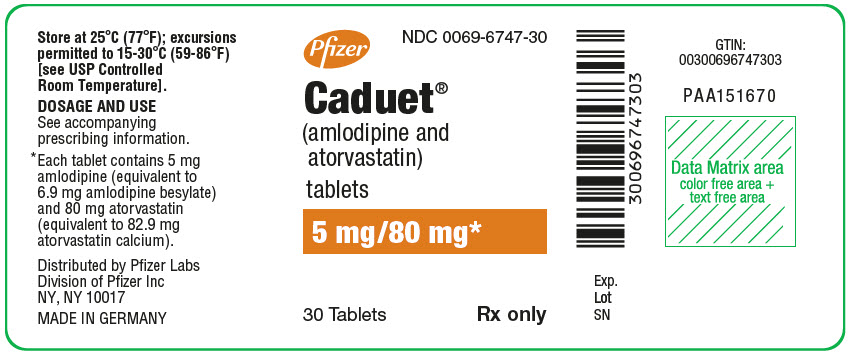
Pfizer
NDC 0069-6747-30
Caduet®
(amlodipine and
atorvastatin)
tablets
5 mg/80 mg*
30 Tablets
Rx only
PRINCIPAL DISPLAY PANEL
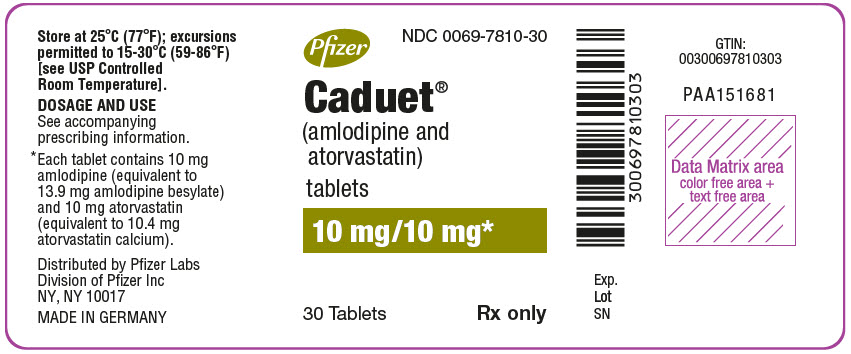
Pfizer
NDC 0069-7810-30
Caduet®
(amlodipine and
atorvastatin)
tablets
10 mg/10 mg*
30 Tablets
Rx only
PRINCIPAL DISPLAY PANEL
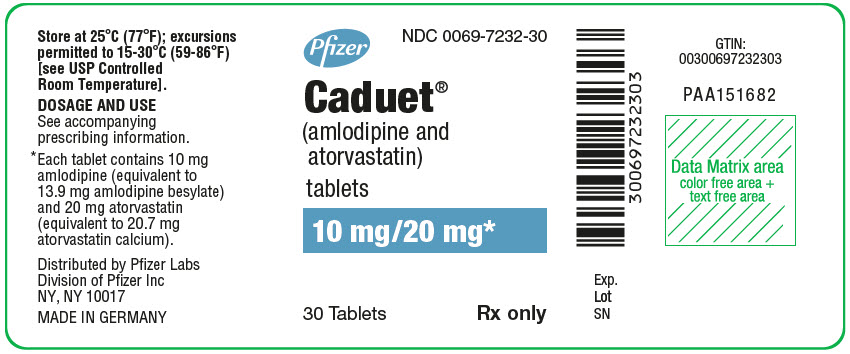
Pfizer
NDC 0069-7232-30
Caduet®
(amlodipine and
atorvastatin)
tablets
10 mg/20 mg*
30 Tablets
Rx only
PRINCIPAL DISPLAY PANEL
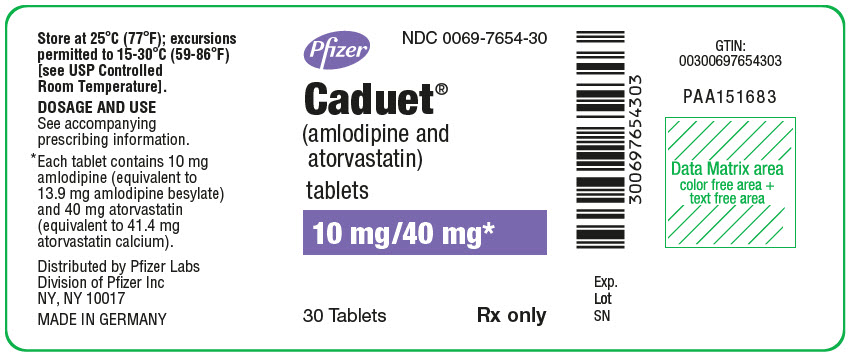
Pfizer
NDC 0069-7654-30
Caduet®
(amlodipine and
atorvastatin)
tablets
10 mg/40 mg*
30 Tablets
Rx only
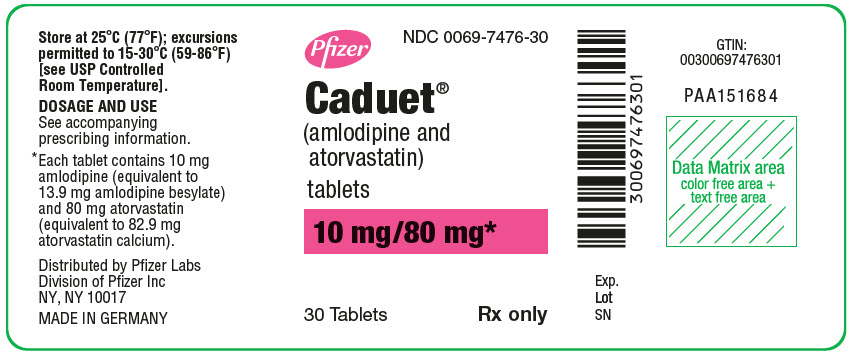
PRINCIPAL DISPLAY PANEL
Pfizer
NDC 0069-7476-30
Caduet®
(amlodipine and
atorvastatin)
tablets
10 mg/80 mg*
30 Tablets
Rx only