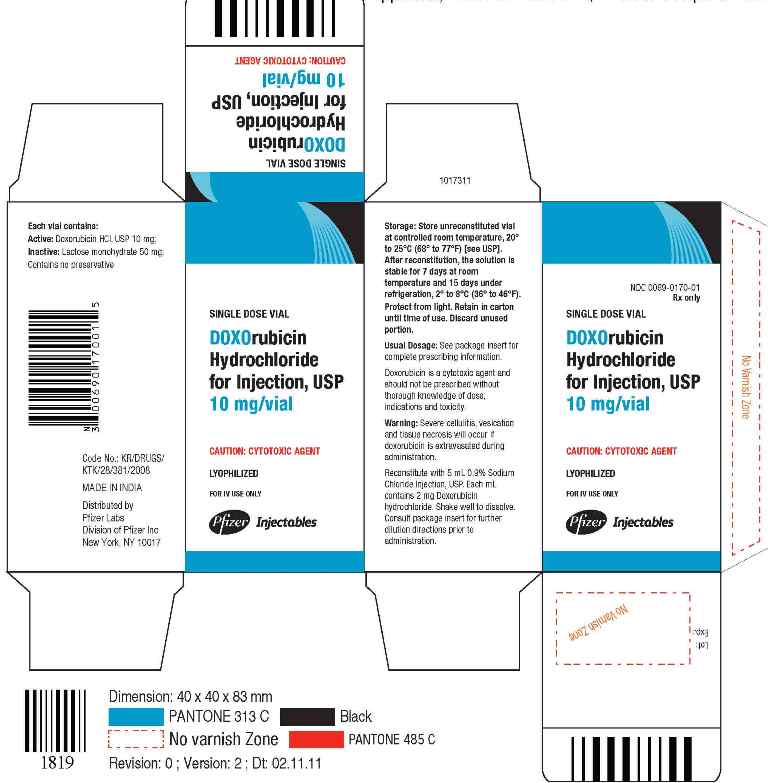
NDC Code(s) : 0069-0170-01, 0069-0171-01
Packager : Pfizer Laboratories Div Pfizer Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Doxorubicin HydrochlorideDoxorubicin Hydrochloride INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
INGREDIENTS AND APPEARANCE
| Doxorubicin HydrochlorideDoxorubicin Hydrochloride INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
PRINCIPAL DISPLAY PANEL
Single Dose Vial
DOXOrubicin Hydrochloride for Injection, USP 50mg/Vial
Caution: Cytotoxic Agent
Lyophilized
For IV use only

Single Dose Vial
DOXOrubicin Hydrochloride for Injection, USP 10mg/Vial
Caution: Cytotoxic Agent
Lyophilized
For IV use only