NDC Code(s) : 0062-0165-01, 0062-0165-02, 0062-0175-12, 0062-0175-13, 0062-0275-23, 0062-0275-01, 0062-0575-44, 0062-0575-46, 0062-0475-42, 0062-0475-45
Packager : Ortho-McNeil Janssen Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Retin-ATretinoin CREAM | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Retin-ATretinoin CREAM | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Retin-ATretinoin CREAM | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Retin-ATretinoin GEL | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Retin-ATretinoin GEL | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
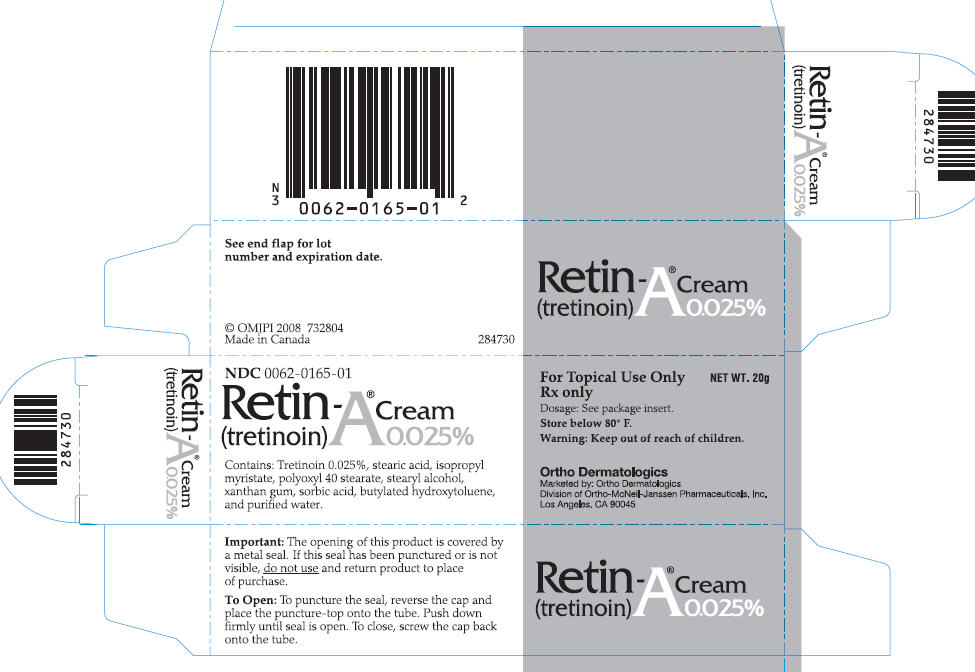
NDC 0062-0165-01
Retin-A® Cream
(tretinoin) 0.025%
Contains: Tretinoin 0.025%, stearic acid, isopropyl
myristate, polyoxyl 40 stearate, stearyl alcohol, xanthan
gum, sorbic acid, butylated hydroxytoluene,
and purified water
For Topical Use only NET WT. 20g
Rx only
Dosage: See package insert.
Store below 80° F.
Warning: Keep out of reach of children.
Ortho Dermatologics
Marketed by: Ortho Dermatologics
Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc.
Los Angeles, CA 90045
PRINCIPAL DISPLAY PANEL
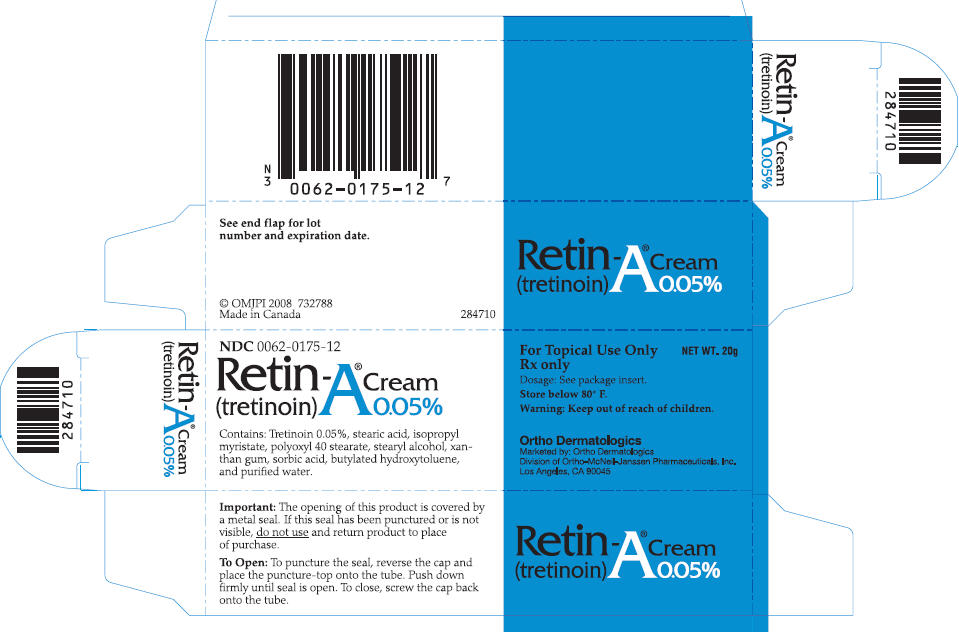
NDC 0062-0175-12
Retin-A® Cream
(tretinoin) 0.05%
Contains: Tretinoin 0.05%, stearic acid, isopropyl
myristate, polyoxyl 40 stearate, stearyl alcohol, xanthan
gum, sorbic acid, butylated hydroxytoluene,
and purified water
For Topical Use only NET WT. 20g
Rx only
Dosage: See package insert.
Store below 80° F.
Warning: Keep out of reach of children.
Ortho Dermatologics
Marketed by: Ortho Dermatologics
Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc.
Los Angeles, CA 90045
PRINCIPAL DISPLAY PANEL
NDC 0062-0275-23
Retin-A® Cream
(tretinoin) 0.1%
Contains: Tretinoin 0.1%, stearic acid, isopropyl
myristate, polyoxyl 40 stearate, stearyl alcohol, xanthan
gum, sorbic acid, butylated hydroxytoluene,
and purified water
For Topical Use only NET WT. 20g
Rx only
Dosage: See package insert.
Store below 80° F.
Warning: Keep out of reach of children.
Ortho Dermatologics
Marketed by: Ortho Dermatologics
Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc.
Los Angeles, CA 90045

PRINCIPAL DISPLAY PANEL
NDC 0062-0575-44
Retin-A® Gel
(tretinoin) 0.01%
Contains: Tretinoin 0.01%, hydroxypropyl cellulose,
butylated hydroxytoluene, alcohol, (denatured with tert-
butyl alcohol and brucine sulfate) 90% w/w.
For topical Use Only NET WT. 15g
Rx only
Dosage: See package insert.
Store below 86° F.
Warning: Keep out of reach of children.
Ortho Dermatologics
Marketed by: Ortho Dermatologics
Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc.
Los Angeles, CA 90045

PRINCIPAL DISPLAY PANEL
NDC 0062-0475-42
Retin-A® Gel
(tretinoin) 0.025%
Contains: Tretinoin 0.01%, hydroxypropyl cellulose,
butylated hydroxytoluene, alcohol, (denatured with tert-
butyl alcohol and brucine sulfate) 90% w/w.
For topical Use Only NET WT. 15g
Rx only
Dosage: See package insert.
Store below 86° F.
Warning: Keep out of reach of children.
Ortho Dermatologics
Marketed by: Ortho Dermatologics
Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc.
Los Angeles, CA 90045










