NDC Code(s) : 0054-4179-25, 0054-4180-25, 0054-4181-25, 0054-4182-25, 0054-4182-31, 0054-4183-25, 0054-4184-25, 0054-4186-25, 0054-3176-44, 0054-3177-57, 0054-3177-63, 0054-8179-25, 0054-8180-25, 0054-8174-25, 0054-8181-25, 0054-8176-25, 0054-8175-25, 0054-8183-25
Packager : Hikma Pharmaceuticals USA, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Dexamethasone IntensolDexamethasone Intensol SOLUTION, CONCENTRATE | ||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| DexamethasoneDexamethasone SOLUTION | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DexamethasoneDexamethasone TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Hikma Pharmaceuticals USA, Inc.(080189610) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| West-Ward Columbus Inc. | 058839929 | MANUFACTURE(0054-4179, 0054-4180, 0054-4181, 0054-4182, 0054-4183, 0054-4184, 0054-4186, 0054-3176, 0054-3177, 0054-8179, 0054-8180, 0054-8174, 0054-8181, 0054-8176, 0054-8175, 0054-8183) | |
PRINCIPAL DISPLAY PANEL
NDC 0054-4179-25 100 Tablets
Dexamethasone Tablets, USP
0.5 mg

PRINCIPAL DISPLAY PANEL
NDC 0054-8179-25 10x10 Unit-Dose Tablets
Dexamethasone Tablets, USP
0.5 mg

PRINCIPAL DISPLAY PANEL
NDC 0054-4180-25 100 Tablets
Dexamethasone Tablets, USP
0.75 mg

PRINCIPAL DISPLAY PANEL
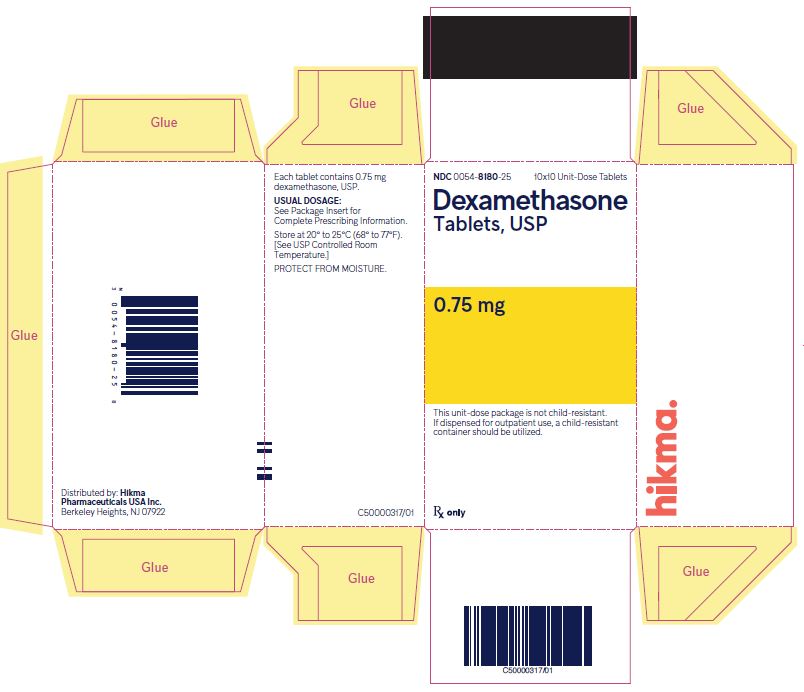
NDC 0054-8180-25 10x10 Unit-Dose Tablets
Dexamethasone Tablets, USP
0.75 mg
PRINCIPAL DISPLAY PANEL
NDC 0054-4181-25 100 Tablets
Dexamethasone Tablets, USP
1 mg

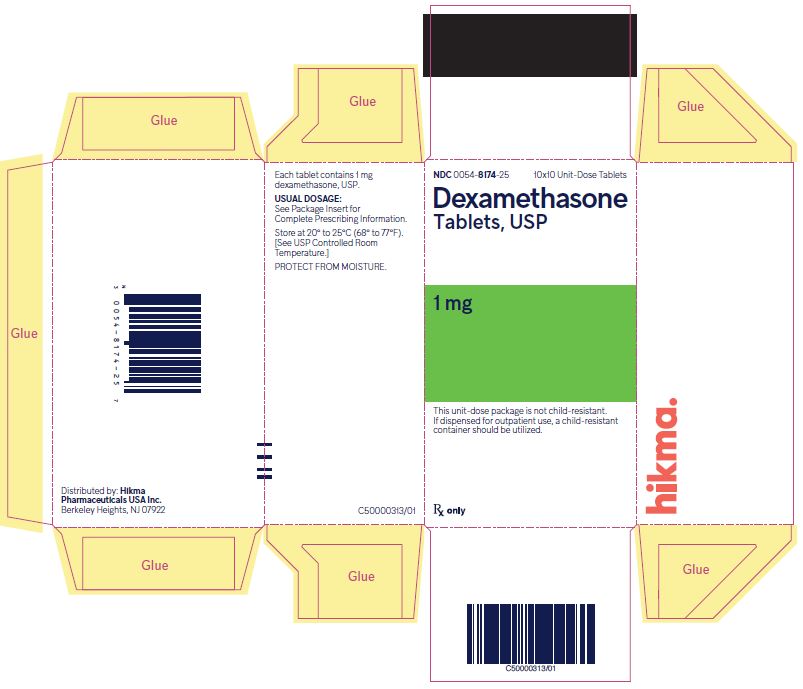
PRINCIPAL DISPLAY PANEL
NDC 0054-8174-25 10x10 Unit-Dose Tablets
Dexamethasone Tablets, USP
1 mg
PRINCIPAL DISPLAY PANEL
NDC 0054-4182-25 100 Tablets
Dexamethasone Tablets, USP
1.5 mg

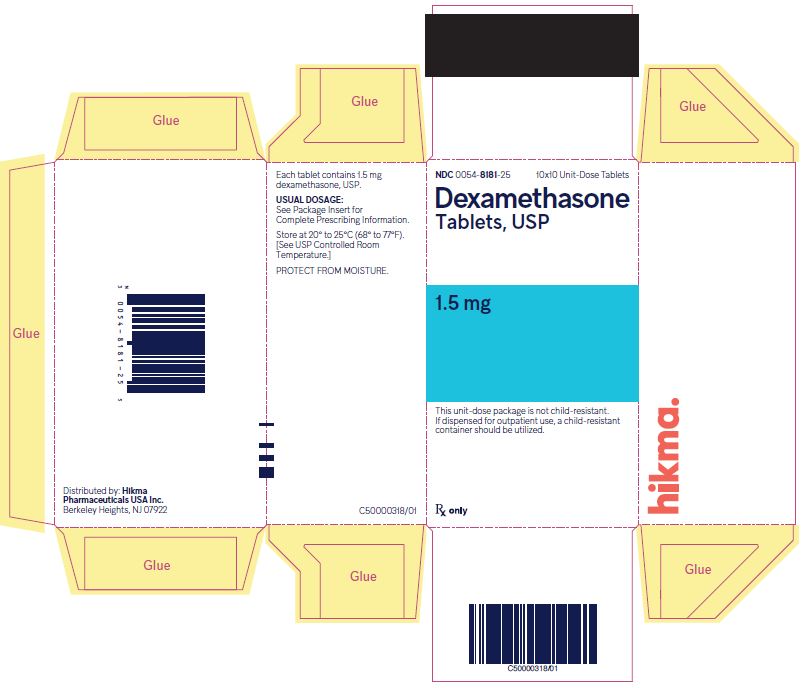
PRINCIPAL DISPLAY PANEL
NDC 0054-8181-25 10x10 Unit-Dose Tablets
Dexamethasone Tablets, USP
1.5 mg
PRINCIPAL DISPLAY PANEL
NDC 0054-4183-25 100 Tablets
Dexamethasone Tablets, USP
2 mg

PRINCIPAL DISPLAY PANEL
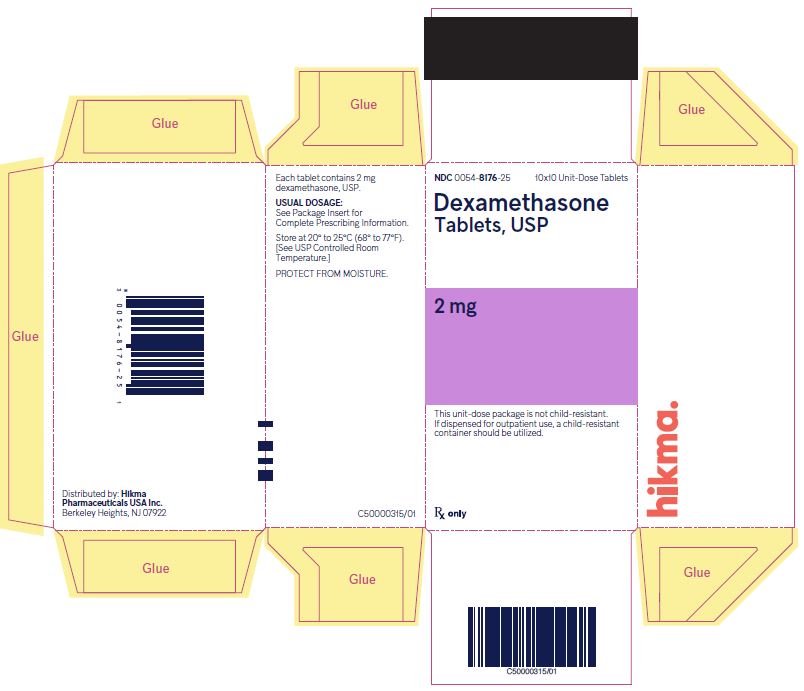
NDC 0054-8176-25 10x10 Unit-Dose Tablets
Dexamethasone Tablets, USP
2 mg
PRINCIPAL DISPLAY PANEL
NDC 0054-4184-25 100 Tablets
Dexamethasone Tablets, USP
4 mg

PRINCIPAL DISPLAY PANEL
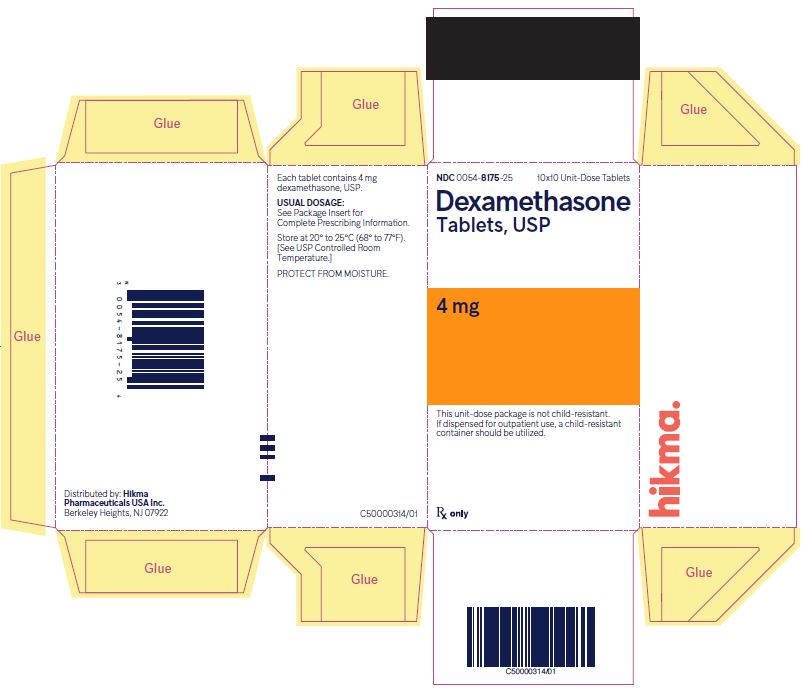
NDC 0054-8175-25 10x10 Unit-Dose Tablets
Dexamethasone Tablets, USP
4 mg
PRINCIPAL DISPLAY PANEL
NDC 0054-4186-25 100 Tablets
Dexamethasone Tablets, USP
6 mg

PRINCIPAL DISPLAY PANEL
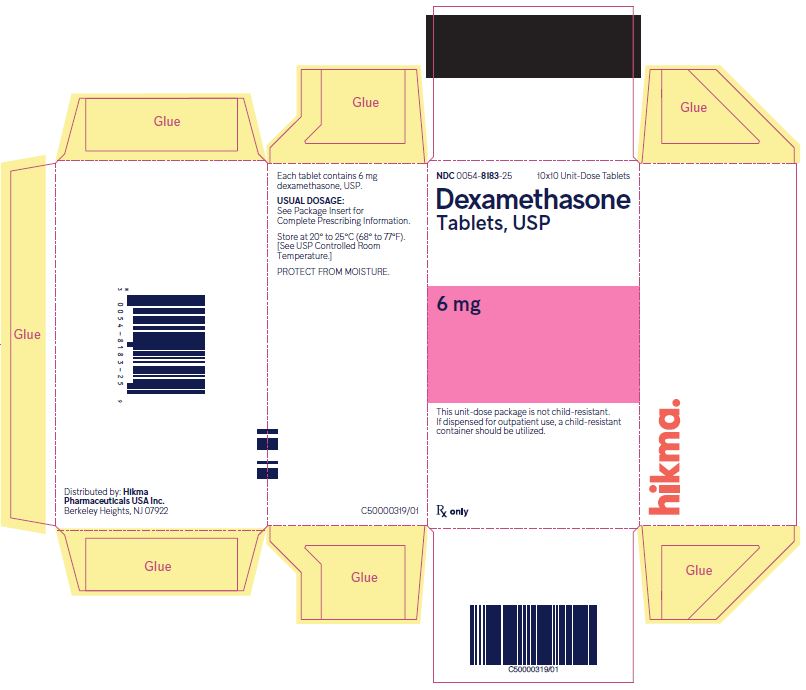
NDC 0054-8183-25 10x10 Unit-Dose Tablets
Dexamethasone Tablets, USP
6 mg
PRINCIPAL DISPLAY PANEL
NDC 0054-3177-57 240 mL
Dexamethasone Oral Solution, USP
0.5 mg per 5 mL

PRINCIPAL DISPLAY PANEL
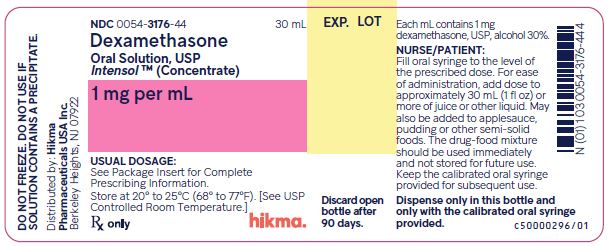
NDC 0054-3176-44 30 mL
Dexamethasone Oral Solution, USP
Intensol™ (Concentrate)
1 mg per mL