NDC Code(s) : 0039-0067-10, 0039-0067-70, 0039-0060-13, 0039-0060-50, 0039-0060-70, 0039-0066-05, 0039-0066-50
Packager : Sanofi-Aventis U.S. LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Lasixfurosemide TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lasixfurosemide TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Lasixfurosemide TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
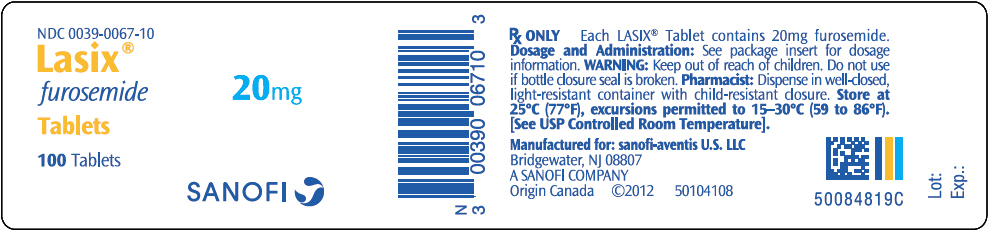
NDC 0039-0067-10
Lasix
®
furosemide
Tablets
100 Tablets
20mg
SANOFI
PRINCIPAL DISPLAY PANEL
NDC 0039-0060-13
Lasix
®
furosemide
Tablets
100 Tablets
40mg
SANOFI

PRINCIPAL DISPLAY PANEL
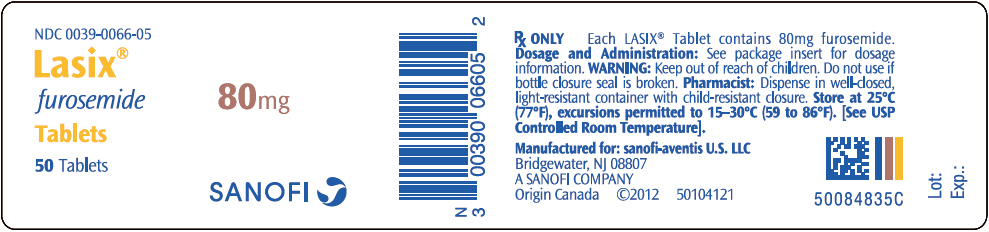
NDC 0039-0066-05
Lasix
®
furosemide
Tablets
50 Tablets
80mg
SANOFI