NDC Code(s) : 0025-1515-01, 0025-1520-31, 0025-1520-51, 0025-1520-34, 0025-1525-31, 0025-1525-51, 0025-1525-34, 0025-1525-99, 0025-1525-96, 0025-1530-02, 0025-1530-01
Packager : PFIZER LABORATORIES DIV PFIZER INC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CELEBREXCelecoxib CAPSULE | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| CELEBREXCelecoxib CAPSULE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CELEBREXCelecoxib CAPSULE | |||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| CELEBREXCelecoxib CAPSULE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - PFIZER LABORATORIES DIV PFIZER INC(134489525) |
PRINCIPAL DISPLAY PANEL
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0025-1515-01
Pfizer
Celebrex
®
celecoxib capsules
50 mg
60 Capsules
Rx only

PRINCIPAL DISPLAY PANEL
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0025-1520-31
Pfizer
Celebrex
®
celecoxib capsules
100 mg
100 Capsules
Rx only

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 mg Blister Pack
Celebrex®
celecoxib capsules
100 mg
Distributed by
G.D. Searle LLC
Div of Pfizer Inc, NY, NY 10017
MADE IN SINGAPORE

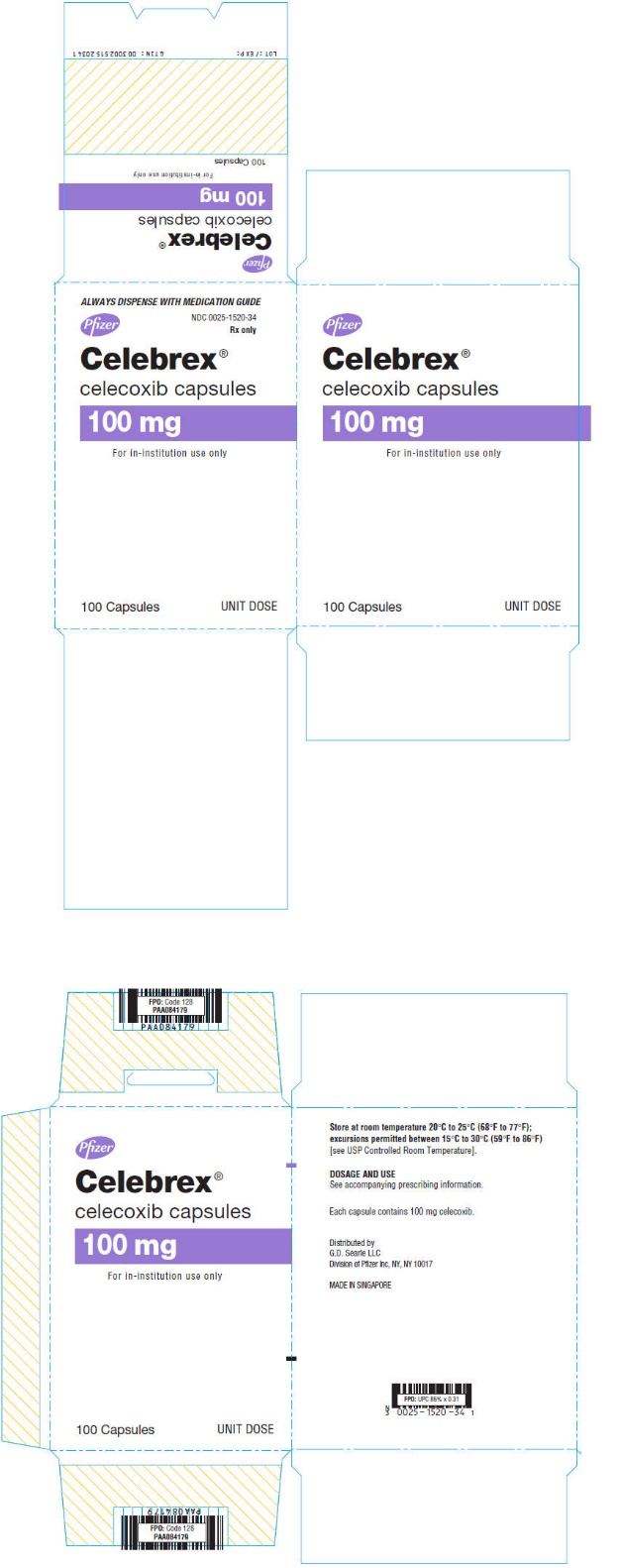
PRINCIPAL DISPLAY PANEL
ALWAYS DISPENSE WITH MEDICATION GUIDE
Pfizer
NDC 0025-1520-34
Rx only
Celebrex®
celecoxib capsules
100 mg
For in-institution use only
100 Capsules
UNIT DOSE
PRINCIPAL DISPLAY PANEL
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0025-1525-31
Pfizer
Celebrex
®
celecoxib capsules
200 mg
100 Capsules
Rx only

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 200 mg Blister Pack
Celebrex®
celecoxib capsules
200 mg
Distributed by
G.D. Searle LLC
Div of Pfizer Inc, NY, NY 10017
MADE IN SINGAPORE

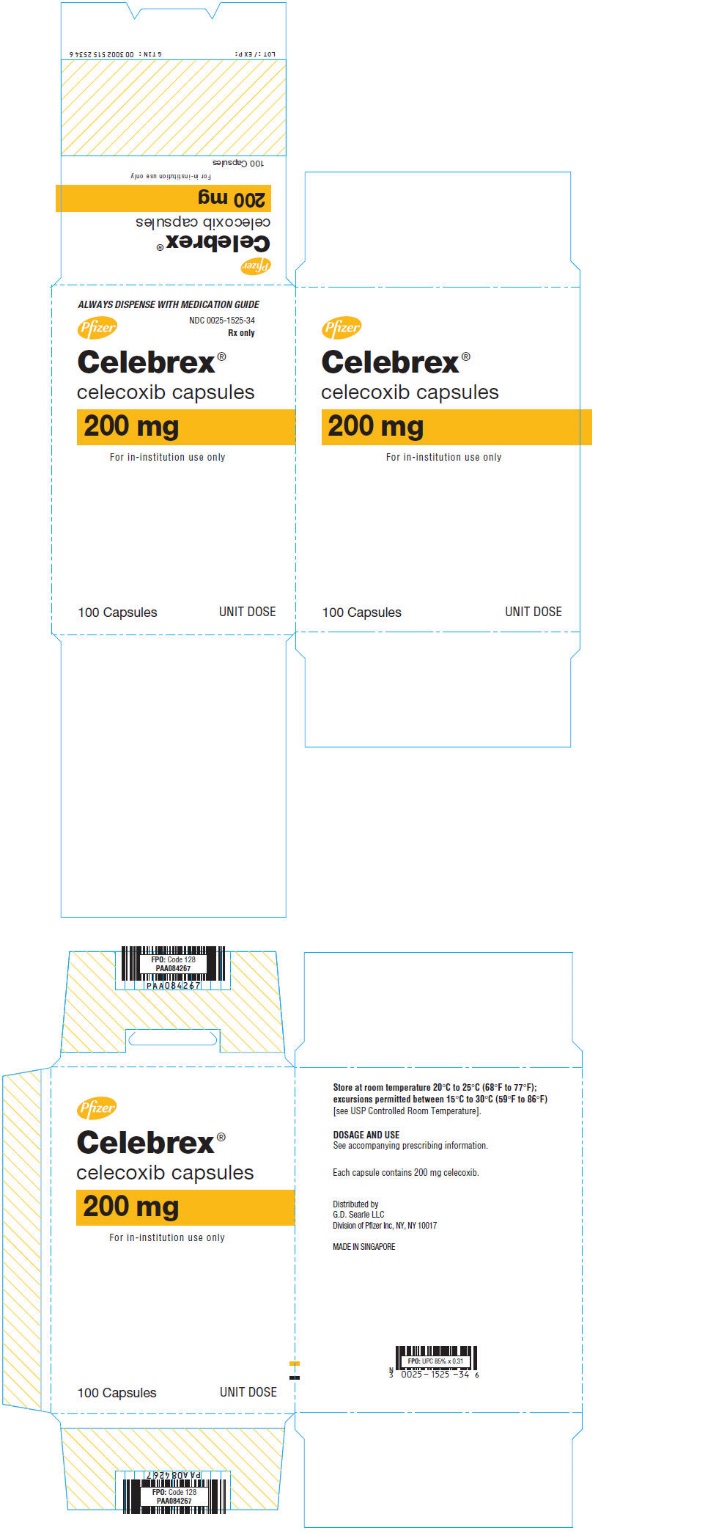
PRINCIPAL DISPLAY PANEL
ALWAYS DISPENSE WITH MEDICATION GUIDE
Pfizer
NDC 0025-1525-34
Rx only
Celebrex®
celecoxib capsules
200 mg
For in-institution use only
100 Capsules
UNIT DOSE
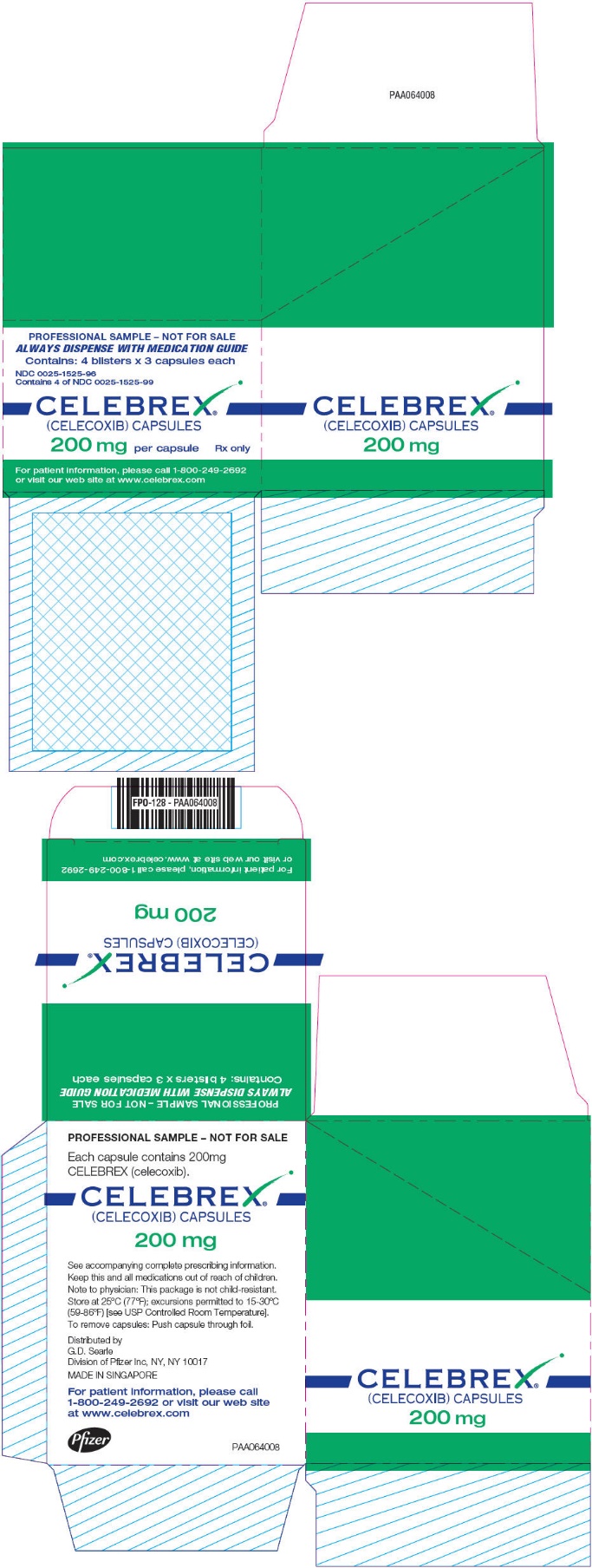
PRINCIPAL DISPLAY PANEL
ALWAYS DISPENSE WITH MEDICATION GUIDE
CELEBREX
®
(CELECOXIB) CAPSULES
200 mg
1-800-249-2692
www.celebrex.com
200 mg
200 mg
200 mg

PRINCIPAL DISPLAY PANEL
NDC 0025-1525-99
Rx only
PROFESSIONAL SAMPLE – NOT FOR SALE
ALWAYS DISPENSE WITH MEDICATION GUIDE
Contains: 1 blister x 3 capsules
CELEBREX
®
(CELECOXIB) CAPSULES
200 mg per capsule
For patient information, please call 1-800-249-2692
or visit our web site at www.celebrex.com

PRINCIPAL DISPLAY PANEL
PROFESSIONAL SAMPLE – NOT FOR SALE
ALWAYS DISPENSE WITH MEDICATION GUIDE
Contains: 4 blisters x 3 capsules each
NDC 0025-1525-96
Contains 4 of NDC 0025-1525-99
CELEBREX
®
(CELECOXIB) CAPSULES
200 mg per capsule
Rx only
For patient information, please call 1-800-249-2692
or visit our web site at www.celebrex.com
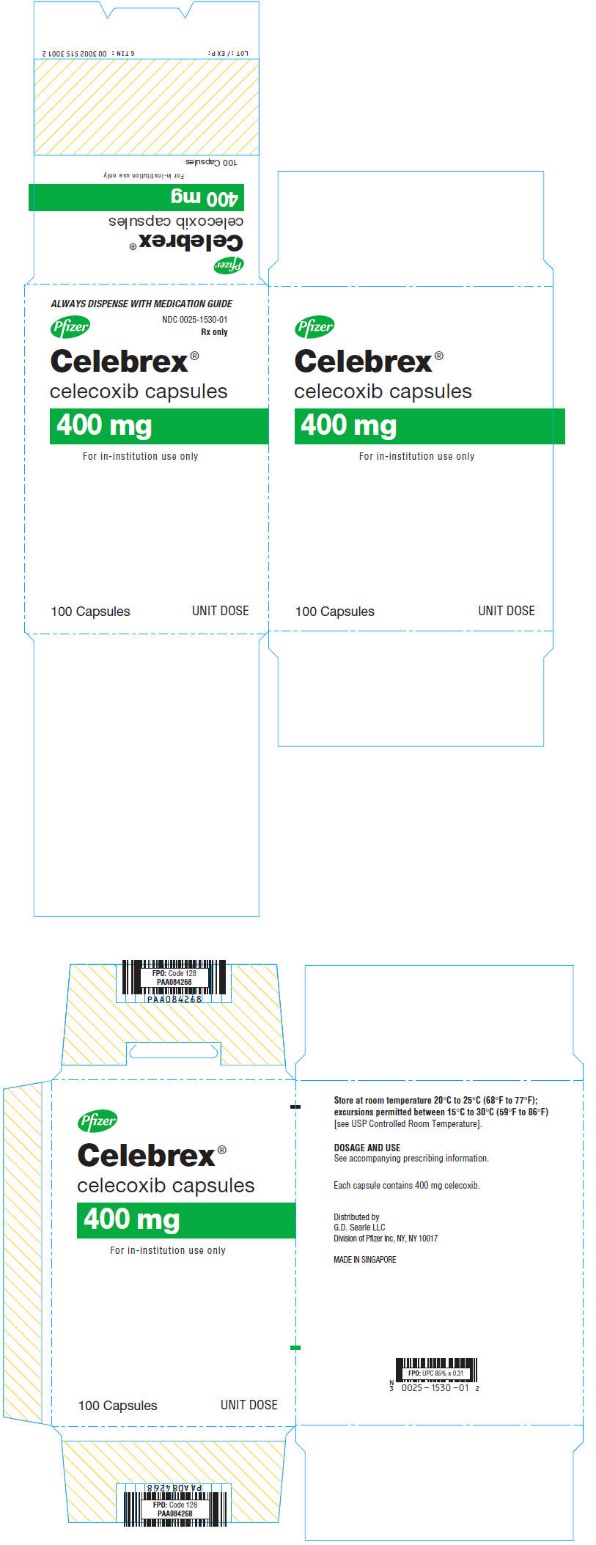
PRINCIPAL DISPLAY PANEL
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0025-1530-02
Pfizer
Celebrex
®
celecoxib capsules
400 mg
60 Capsules
Rx only

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 400 mg Blister Pack
Celebrex®
celecoxib capsules
400 mg
Distributed by
G.D. Searle LLC
Div of Pfizer Inc, NY, NY 10017
MADE IN SINGAPORE

PRINCIPAL DISPLAY PANEL
ALWAYS DISPENSE WITH MEDICATION GUIDE
Pfizer
NDC 0025-1530-01
Rx only
Celebrex®
celecoxib capsules
400 mg
For in-institution use only
100 Capsules
UNIT DOSE