NDC Code(s) : 0015-1910-12, 0015-1911-13
Packager : E.R. Squibb & Sons, L.L.C.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| IXEMPRAixabepilone KIT | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| IXEMPRAixabepilone KIT | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
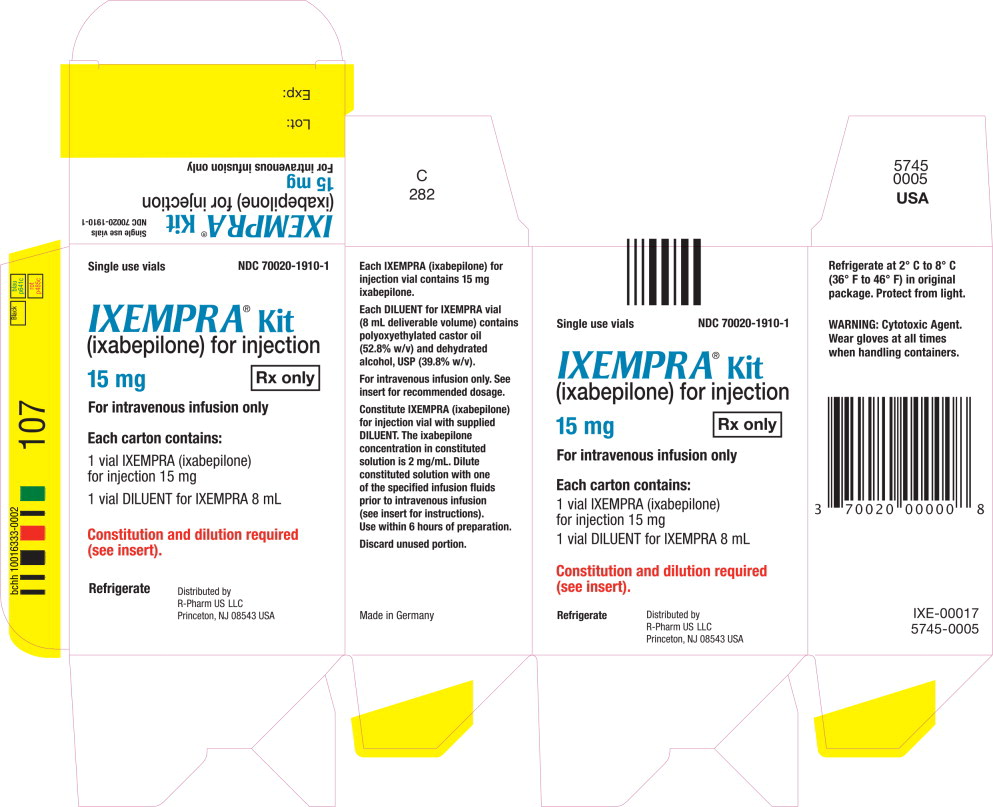
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Carton Label
Single use vials NDC 70020-1910-1
IXEMPRA ® Kit
(ixabepilone) for injection
15 mg Rx only
For intravenous infusion only
Each carton contains
1 vial IXEMPRA (ixabepilone)
for injection 15 mg
1 vial DILUENT for IXEMPRA 8 mL
Constitution and dilution required
(see insert).
Refrigerate
Distribute by
R-Pharm US LLC
Princeton, NJ 08543 USA
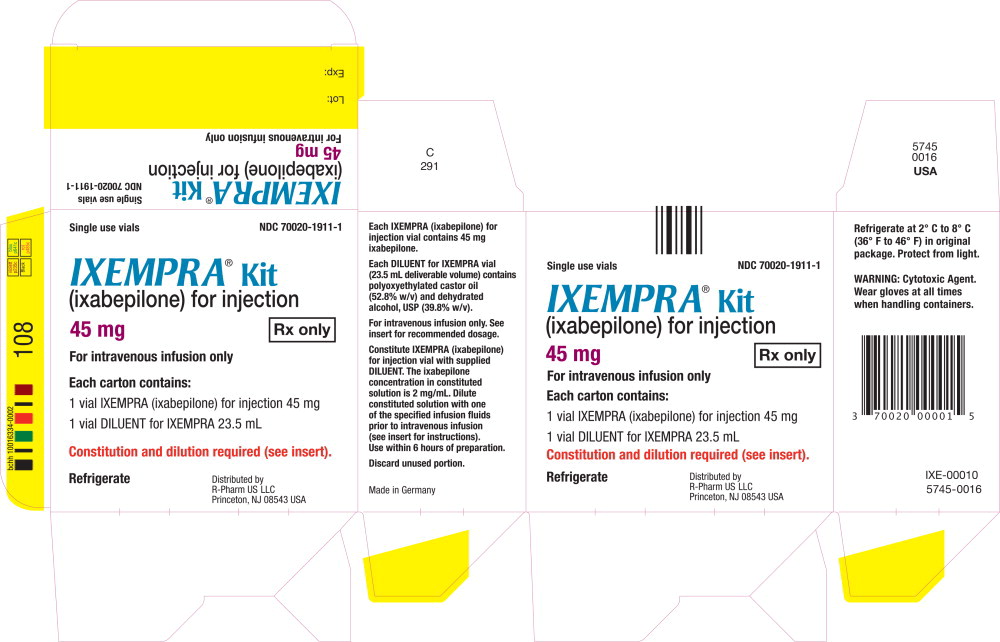
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Carton Label
Single use vials NDC 70020-1911-1
IXEMPRA ® Kit
(ixabepilone) for injection
45 mg Rx only
For intravenous infusion only
Each carton contains
1 vial IXEMPRA (ixabepilone)
for injection 15 mg
1 vial DILUENT for IXEMPRA 23.5 mL
Constitution and dilution required (see insert).
Refrigerate
Distribute by
R-Pharm US LLC
Princeton, NJ 08543 USA
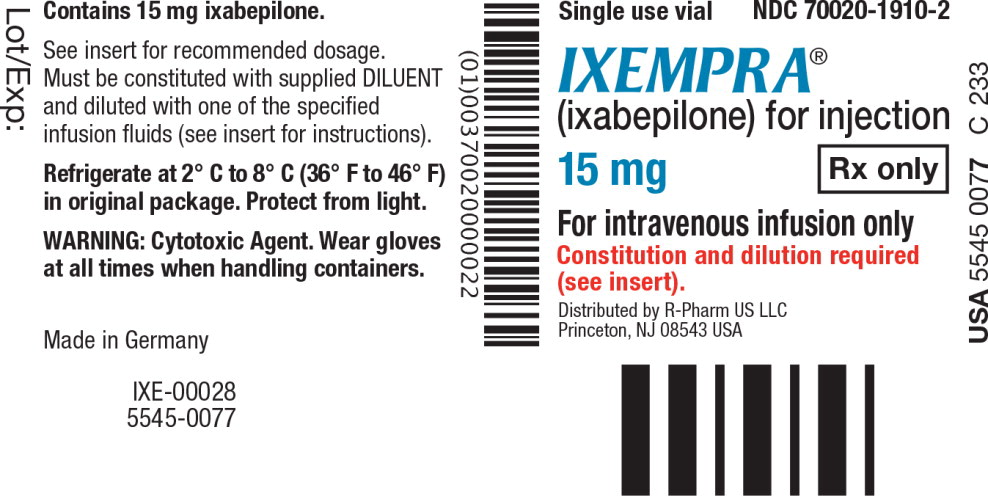
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Vial Label
Single use vial NDC 70020-1910-2
IXEMPRA
®
(ixabepilone) for injection
15 mg Rx only
For intravenous infusion only
Constitution and dilution required
(see insert).
Distributed by R-Pharm US LLC
Princeton, NJ 08543 USA
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Vial Label
Single use vial NDC 70020-1911-2
IXEMPRA
®
(ixabepilone) for injection
45 mg Rx only
For intravenous infusion only
Constitution and dilution required
(see insert).
Distributed by R-Pharm US LLC
Princeton, NJ 08543 USA

PRINCIPAL DISPLAY PANEL
Principal Display Panel - Vial Label
Single use vial NDC 70020-1265-1
DILUENT for
IXEMPRA
®
(ixabepilone) for injection
8 mL Rx only
Not for direct administration
Distributed by R-Pharm US LLC
Princeton, NJ 08543 USA

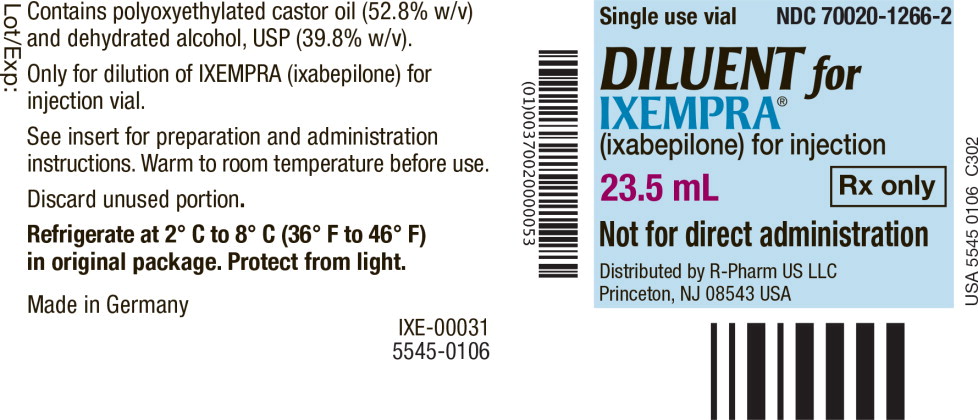
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Vial Label
Single use vial NDC 70020-1266-2
DILUENT for
IXEMPRA
®
(ixabepilone) for injection
23.5 mL Rx only
Not for direct administration
Distributed by R-Pharm US LLC
Princeton, NJ 08543 USA