NDC Code(s) : 0006-3931-10, 0006-3931-30, 0006-3931-54, 0006-3931-36
Packager : Merck Sharp & Dohme Corp.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ZIOPTANTAFLUPROST SOLUTION | |||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
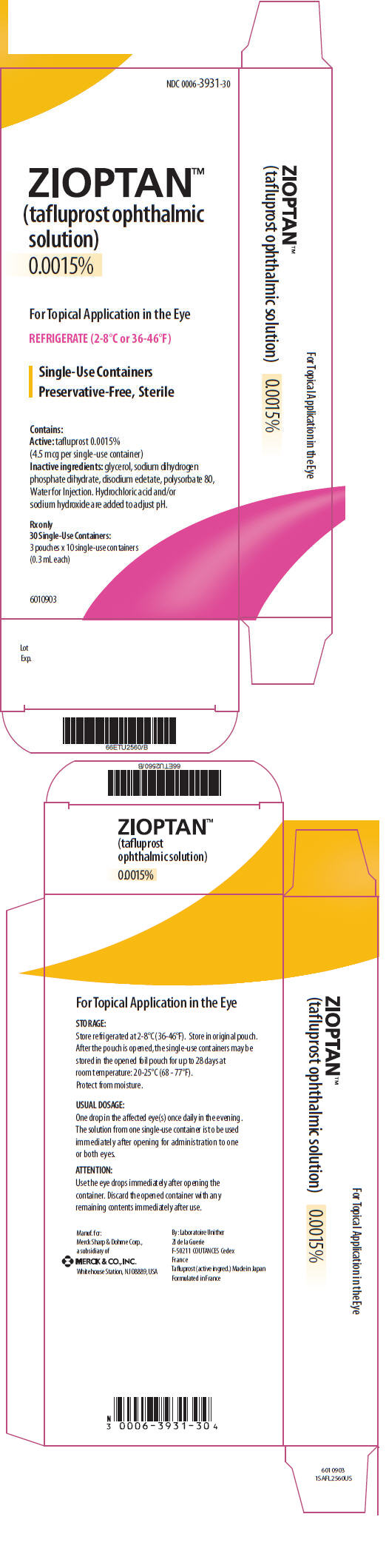
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 30 Ampule Carton
NDC 0006-3931-30
ZIOPTAN™
(tafluprost ophthalmic
solution)
0.0015%
For Topical Application in the Eye
REFRIGERATE (2-8°C or 36-46°F)
Single-Use Containers
Preservative-Free, Sterile
Contains:
Active: tafluprost 0.0015%
(4.5 mcg per single-use container)
Inactive ingredients: glycerol, sodium dihydrogen
phosphate dihydrate, disodium edetate, polysorbate 80,
Water for Injection. Hydrochloric acid and/or
sodium hydroxide are added to adjust pH.
Rx only
30 Single-Use Containers:
3 pouches × 10 single-use containers
(0.3 mL each)
6010903