

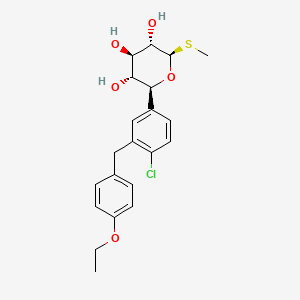

1. (2s,3r,4r,5s,6r)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(methylthio)tetrahydro-2h-pyran-3,4,5-triol
2. Lx-4211
3. Lx4211
1. 1018899-04-1
2. Lx-4211
3. Lx4211
4. Lp-802034
5. (2s,3r,4r,5s,6r)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(methylthio)tetrahydro-2h-pyran-3,4,5-triol
6. 6b4zbs263y
7. Sar439954
8. Sar-439954
9. (2s,3r,4r,5s,6r)-2-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6-methylsulfanyloxane-3,4,5-triol
10. Zynquista
11. Beta-l-xylopyranoside, Methyl 5-c-(4-chloro-3-((4-ethoxyphenyl)methyl)phenyl)-1-thio-, (5s)-
12. Sotagliflozin [usan:inn]
13. Unii-6b4zbs263y
14. Beta-l-xylopyranoside, Methyl 5-c-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-1-thio-, (5s)-
15. Mfcd22493506
16. Lx 4211
17. Lp 802034
18. Sotagliflozin [mi]
19. Sotagliflozin (lx4211)
20. Sotagliflozin [inn]
21. Sotagliflozin (usan/inn)
22. Sotagliflozin [usan]
23. Sotagliflozin (lx-4211)
24. Sotagliflozin [who-dd]
25. Gtpl8312
26. Schembl1038287
27. Chembl3039507
28. Dtxsid20144314
29. Amy12393
30. Ex-a1167
31. Bdbm50235017
32. S8103
33. Zinc95641922
34. Akos025290846
35. Ccg-268940
36. Cs-1069
37. Db12713
38. Ac-29890
39. As-35202
40. Bl161424
41. Hy-15516
42. D10669
43. A852129
44. Q27088840
45. (2s,3r,4r,5s,6r)-2-(3-(4-ethoxybenzyl)-4-chlorophenyl)-tetrahydro-6-(methylthio)-2h-pyran-3,4,5-triol;lx4211
46. (2s,3r,4r,5s,6r)-2-[4-chloro-3-(4-ethoxybenzyl)phenyl]-6-(methylsulfanyl)tetrahydro-2h-pyran-3,4,5-triol
47. .beta.-l-xylopyranoside, Methyl 5-c-(4-chloro-3-((4-ethoxyphenyl)methyl)phenyl)-1-thio-, (5s)-
48. Methyl (5s)-5-c-(4-chloro-3-((4-ethoxyphenyl)methyl)phenyl)-1-thio-.beta.-l-xylopyranoside
| Molecular Weight | 424.9 g/mol |
|---|---|
| Molecular Formula | C21H25ClO5S |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 424.1111228 g/mol |
| Monoisotopic Mass | 424.1111228 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 476 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Zynquista is indicated as an adjunct to insulin therapy to improve glycaemic control in adults with type 1 diabetes mellitus with a Body Mass Index (BMI) 27 kg/m2, who have failed to achieve adequate glycaemic control despite optimal insulin therapy.
Treatment of type II diabetes mellitus
Treatment of type I diabetes mellitus
Sodium-Glucose Transporter 2 Inhibitors
Compounds that inhibit SODIUM-GLUCOSE TRANSPORTER 2. They lower blood sugar by preventing the reabsorption of glucose by the kidney and are used in the treatment of TYPE 2 DIABETES MELLITUS. (See all compounds classified as Sodium-Glucose Transporter 2 Inhibitors.)
A10
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BK - Sodium-glucose co-transporter 2 (sglt2) inhibitors
A10BK06 - Sotagliflozin
