


1. Molevac
2. Pamoxan
3. Povanyl
4. Pyrcon
5. Pyrvinium Iodide
6. Pyrvinium Monohydroxide
7. Pyrvinium Pamoate
8. Pyrvinium Pamoate (2:1)
9. Vankin
10. Vanquin
1. 7187-62-4
2. Pyrvinum
3. Pyrvinium (cation)
4. Pyrvinium Ion
5. Pyrvinium Cation
6. Pyrvinum (base)
7. Hsdb 3178
8. Chebi:8687
9. 6b9991flu3
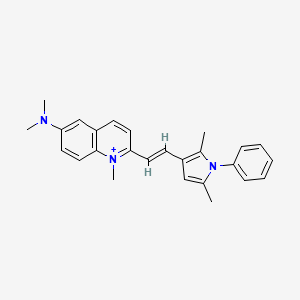
10. 2-[(e)-2-(2,5-dimethyl-1-phenylpyrrol-3-yl)ethenyl]-n,n,1-trimethylquinolin-1-ium-6-amine
11. Quinolinium, 6-(dimethylamino)-2-(2-(2,5-dimethyl-1-phenyl-1h-pyrrol-3-yl)ethenyl)-1-methyl-
12. Pyrvinium-pamoate
13. Pyrvinium [hsdb]
14. 6-(dimethylamino)-2-(2-(2,5-dimethyl-1-phenyl-1h-pyrrol-3-yl)ethenyl)-1-methylquinolinium
15. Unii-6b9991flu3
16. Chembl1201303
17. Dtxsid2043795
18. Zinc3831401
19. Db06816
20. Quinolinium, 6-(dimethylamino)-2-(2-(2,5-dimethyl-1-phenylpyrrol-3-yl)vinyl)-1-methyl-
21. C07412
22. Ab00053809_02
23. Q264039
24. 2-[(e)-2-(2,5-dimethyl-1-phenyl-pyrrol-3-yl)vinyl]-n,n,1-trimethyl-quinolin-1-ium-6-amine
25. 6-(dimethylamino)-2-(2-(2,5-dimethyl-1-phenyl-1h-pyrrol-3-yl)vinyl)-1-methylquinolinium
26. 6-(dimethylamino)-2-[(e)-2-(2,5-dimethyl-1-phenyl-1h-pyrrol-3-yl)ethenyl]-1-methylquinolinium
| Molecular Weight | 382.5 g/mol |
|---|---|
| Molecular Formula | C26H28N3+ |
| XLogP3 | 5.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 4 |
| Exact Mass | 382.228322906 g/mol |
| Monoisotopic Mass | 382.228322906 g/mol |
| Topological Polar Surface Area | 12 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 1 |
| Complexity | 552 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anthelmintics; Fluorescent Dyes
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
FOR CONTROL OF PINWORM INFESTATION IN CHILDREN & ADULTS PYRVINIUM PAMOATE IS GIVEN ORALLY IN SINGLE DOSE ... SECOND DOSE SHOULD BE ADMIN 2 WK LATER, TO ELIM WORMS THAT HAVE DEVELOPED FROM OVA INGESTED AFTER FIRST DOSE. /PAMOATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1030
...OCCUPANTS OF ENTIRE HOUSEHOLD MAY BE TREATED RATHER THAN INVESTIGATING EACH MEMBER BY ANAL SWABS. INVESTIGATIONS ON EFFECT OF THIS DRUG ON STRONGYLOIDIASIS HAVE SHOWN PROMISING RESULTS. ... BECAUSE OF STAINING QUALITY OF DRUG, TABLETS SHOULD BE SWALLOWED IMMEDIATELY WITHOUT CHEWING.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1030
PARENTS & PT SHOULD BE INFORMED THAT DRUG WILL COLOR STOOLS BRIGHT RED, & THAT SUSPENSION, IF SPILLED, WILL STAIN.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1030
For more Therapeutic Uses (Complete) data for PYRVINIUM (7 total), please visit the HSDB record page.
VET: ALTHOUGH EQUALLY EFFECTIVE, CHLORIDE FORM IS 4 TIMES AS TOXIC AS PAMOATE FORM. /CHLORIDE; PAMOATE/
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 501
A CASE OF ACCIDENTAL PARENTERAL INJECTION OF POVAN IS DESCRIBED.
SILVERMAN ET AL; A CASE OF ACCIDENTAL PARENTERAL INJECTION OF POVAN; TOXICOL APPL PHARMACOL 16 (3): 740 (1970)
Pyrvinium was once used in the treatment of pinworm infestations.
Pyrvinium is an anthelmintic agent which acts to kill pinworms.
Anthelmintics
Agents that kill parasitic worms. They are used therapeutically in the treatment of HELMINTHIASIS in man and animal. (See all compounds classified as Anthelmintics.)
P - Antiparasitic products, insecticides and repellents
P02 - Anthelmintics
P02C - Antinematodal agents
P02CX - Other antinematodals
P02CX01 - Pyrvinium
Absorption
Pyrvinium is not significantly absorbed from the gastrointestinal tract.
WHEN GIVEN BY ORAL ROUTE, PYRVINIUM PAMOATE IS NOT ABSORBED FROM GI TRACT TO ANY APPRECIABLE EXTENT. /PAMOATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1030
Pyrvinium is believed to interfere with glucose uptake by pinworms. Pyrvinium is also thought to inhibit mitochondrial respiration complex 1 and suppress the unfolded protein response. It is also believed to supress the Wnt pathway by activating casein kinase 1. These properties have led to the investigation of pyrvinium's activity against cancers like intestinal polyposis.
INHIBITION OF OXYGEN UPTAKE OF ADULT LITOMOSOIDES IS EFFECTED BY...COMPD CONTAINING AMIDINIUM ION SYSTEM, IN WHICH A QUATERNARY NITROGEN IS SEPARATED FROM A TERTIARY NITROGEN BY RESONATING CARBON CHAIN OF ALTERNATING DOUBLE & SINGLE BONDS. THIS RESP INHIBITION IS ASSOC WITH COMPENSATORY INCR IN AEROBIC GLYCOLYSIS. /PAMOATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1029
...ANTHELMINTIC ACTIVITY...IS ASSOC WITH INHIBITION OF RESP IN AEROBES, & INTERFERENCE WITH ABSORPTION OF EXOGENOUS GLUCOSE IN INTESTINAL HELMINTHS. SUCH INTERFERENCE MAY ACCOUNT FOR ANTHELMINTIC EFFECTS OF CYANINES IN TRICHURIASIS & IN OTHER INTESTINAL HELMINTHIASES. /PAMOATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1030
