

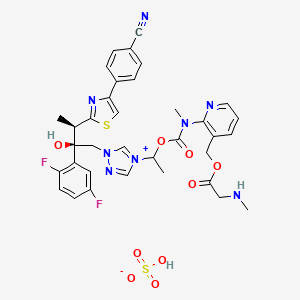

1. 1-((2r,3r)-3-(4-(4-cyanophenyl)-1,3-thiazol-2-yl)-2-(2,5-difluorophenyl)-2-hydroxybutyl)-4-((1rs)-1-((methyl(3-((((methylamino)acetyl)oxy)methyl)pyridin-2-yl)carbamoyl)oxy)ethyl)-1h-1,2,4-triazol-4-ium Monosulfate
2. 4-(2-((2r,3r)-3-(2,5-difluorophenyl)-3-hydroxy-4-(1h-1,2,4-triazol-1-yl)butan-2-yl)-1,3-thiazol-4-yl)benzonitrile
3. Ak-1820
4. Ak1820
5. Bal 4815
6. Bal 8557
7. Bal-4815
8. Bal-8557
9. Bal-8557-002
10. Bal4815
11. Bal8557
12. Bal8557-002
13. Cresemba
14. Isavuconazole
1. 946075-13-4
2. Cresemba
3. Isavuconazonium (sulfate)
4. Bal-8557-002
5. Isavuconazonium Sulfate [usan]
6. Chebi:85977
7. Ak1820
8. 31q44514jv
9. Ak-1820
10. Bal8557-002
11. [2-[1-[1-[(2r,3r)-3-[4-(4-cyanophenyl)-1,3-thiazol-2-yl]-2-(2,5-difluorophenyl)-2-hydroxybutyl]-1,2,4-triazol-4-ium-4-yl]ethoxycarbonyl-methylamino]pyridin-3-yl]methyl 2-(methylamino)acetate;hydrogen Sulfate
12. 1-((2r,3r)-3-(4-(4-cyanophenyl)-1,3-thiazol-2-yl)-2-(2,5-difluorophenyl)-2-hydroxybutyl)-4-((1rs)-1-((methyl(3-((((methylamino)acetyl)oxy)methyl)pyridin-2-yl)carbamoyl)oxy)ethyl)-1h-1,2,4-triazol-4-ium Monosulfate
13. Glycine, N-methyl-, (2-(((1-(1-((2r,3r)-3-(4-(4-cyanophenyl)-2-thiazolyl)-2-(2,5-difluorophenyl)-2-hydroxybutyl)-4h-1,2,4-triazolium-4-yl)ethoxy)carbonyl)methylamino)-3-pyridinyl)methyl Ester, Sulfate (1:1)
14. Isavuconazonium Sulfate (usan)
15. 1-((2r,3r)-3-(4-(4-cyanophenyl)thiazol-2-yl)-2-(2,5-difluorophenyl)-2-hydroxybutyl)-4-(1-((methyl(3-(((methylglycyl)oxy)methyl)pyridin-2-yl)carbamoyl)oxy)ethyl)-1h-1,2,4-triazol-4-ium Hydrogen Sulfate
16. Unii-31q44514jv
17. Chembl3137333
18. Dtxsid901026216
19. Ex-a1786
20. Isavuconazonium Sulfate [jan]
21. Akos030527357
22. Isavuconazonium Sulfate [who-dd]
23. Ac-31126
24. As-35043
25. Hy-100373
26. Cs-0018702
27. Isavuconazonium Sulfate [orange Book]
28. D10643
29. A900138
30. Q27158829
31. (2-{[(1-{1-[(2r,3r)-3-[4-(4-cyanophenyl)-1,3-thiazol-2-yl]-2-(2,5-difluorophenyl)-2-hydroxybutyl]-1h-1,2,4-triazol-4-ium-4-yl}ethoxy)carbonyl](methyl)amino}pyridin-3-yl)methyl N-methylglycinate Hydrogen Sulfate
| Molecular Weight | 814.8 g/mol |
|---|---|
| Molecular Formula | C35H36F2N8O9S2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 17 |
| Rotatable Bond Count | 15 |
| Exact Mass | 814.20147343 g/mol |
| Monoisotopic Mass | 814.20147343 g/mol |
| Topological Polar Surface Area | 273 Ų |
| Heavy Atom Count | 56 |
| Formal Charge | 0 |
| Complexity | 1290 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Treatment of invasive aspergillosis, Treatment of mucormycosis
Treatment of Candida infections
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)

