


1. Recinnamine
2. Reserpinene
3. Trimethoxycinnamoyl Methyl Reserpate
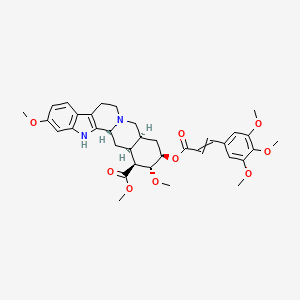
4. Methyl (1r,15s,17r,18r,19s,20s)-6,18-dimethoxy-17-[3-(3,4,5-trimethoxyphenyl)prop-2-enoyloxy]-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylate
5. Ncgc00016787-01
6. 3,4,5-trimethoxycinnamoyl Methyl Reserpate
7. Cas-24815-24-5
8. Prestwick0_000568
9. Prestwick1_000568
10. Dsstox_cid_3554
11. Dsstox_rid_77079
12. Dsstox_gsid_23554
13. Spbio_002575
14. Chembl3182071
15. Tox21_110611
16. Db01180
17. Q27164665
18. (1r,15s,17r,18r,19s,20s)-6,18-dimethoxy-17-[1-oxo-3-(3,4,5-trimethoxyphenyl)prop-2-enoxy]-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylic Acid Methyl Ester
| Molecular Weight | 634.7 g/mol |
|---|---|
| Molecular Formula | C35H42N2O9 |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 11 |
| Exact Mass | 634.28903092 g/mol |
| Monoisotopic Mass | 634.28903092 g/mol |
| Topological Polar Surface Area | 118 Ų |
| Heavy Atom Count | 46 |
| Formal Charge | 0 |
| Complexity | 1080 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 1 |
| Covalently Bonded Unit Count | 1 |
For the treatment of hypertension.
Used to treat hypertension. Rescinnamine inhibits angiotensin-converting enzyme. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex and general vasoconstriction, both of which lead to increases vascular resistance. By inhibiting angiotensin II, aldosterone reabsorption is decreased as well as vasoconstriction. This combined effect serves to decrease blood pressure.
Rescinnamine Binds to and inhibits the angiotensin converting enzyme. Rescinnamine competes with angiotensin I for binding at the angiotensin-converting enzyme, blocking the conversion of angiotensin I to angiotensin II. Inhibition of ACE results in decreased plasma angiotensin II. As angiotensin II is a vasoconstrictor and a negative-feedback mediator for renin activity, lower concentrations result in a decrease in blood pressure and stimulation of baroreceptor reflex mechanisms, which leads to decreased vasopressor activity and to decreased aldosterone secretion.
