

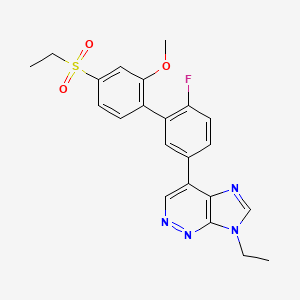

1. 7-ethyl-4-(3-(4-ethylsulfonyl-2-methoxyphenyl)-4-fluorophenyl)imidazo(4,5-c)pyridazine
2. Pf-06372865
1. Pf-06372865
2. 1614245-70-3
3. Darigabat [inn]
4. Darigabat [usan]
5. Cvl-865
6. O9bp19hz3q
7. 7-ethyl-4-(4'-(ethylsulfonyl)-6-fluoro-2'-methoxybiphenyl-3-yl)-7h-imidazo[4,5-c]pyridazine
8. 7-ethyl-4-[3-(4-ethylsulfonyl-2-methoxyphenyl)-4-fluorophenyl]imidazo[4,5-c]pyridazine
9. 7h-imidazo(4,5-c)pyridazine, 7-ethyl-4-(4'-(ethylsulfonyl)-6-fluoro-2'-methoxy(1,1'-biphenyl)-3-yl)-
10. 7-ethyl-4-[4'-(ethylsulfonyl)-6-fluoro-2'-methoxybiphenyl-3-yl]-7h-imidazo[4,5-c]pyridazine
11. Unii-o9bp19hz3q
12. Gtpl9798
13. Chembl3647536
14. Schembl15794150
15. Bdbm144227
16. Tq0078
17. Who 11498
18. Example 4 [wo2014091368]
19. Bp166580
20. Hy-120874
21. Cs-0079452
22. Pf06372865
23. Pf 06372865
24. Us8952008, 4
25. 7-ethyl-4-(4'-(ethylsulfonyl)-6-fluoro-2'-methoxy-[1,1'-biphenyl]-3-yl)-7h-imidazo[4,5-c]pyridazine
26. 7-ethyl-4-(4'-(ethylsulfonyl)-6-fluoro-2'-methoxybiphenyl-3-yl)-7h-imidazo(4,5-c)-pyridazine
27. 7-ethyl-4-(4'-(ethylsulfonyl)-6-fluoro-2'methoxybiphenyl-3-yl)-7h-imidazo[4,5-c]pyridazine
| Molecular Weight | 440.5 g/mol |
|---|---|
| Molecular Formula | C22H21FN4O3S |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Exact Mass | 440.13183988 g/mol |
| Monoisotopic Mass | 440.13183988 g/mol |
| Topological Polar Surface Area | 95.4 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 705 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
