
1. Artesunic Acid
1. Artesunate
2. 88495-63-0
3. Usd5x0z99i
4. Cpd000466336
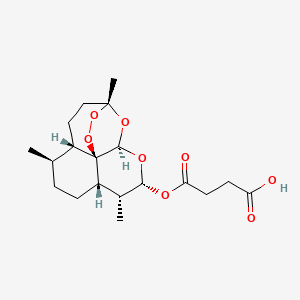
5. Butanedioic Acid,mono[(3r,5as,6r,8as,9r,10s,12r,12ar)-decahydro-3,6,9-trimethyl-3,12-epoxy-12h-pyrano[4,3-j]-1,2-benzodioxepin-10-yl] Ester
6. 182824-33-5
7. Smr000466336
8. Sr-01000763413
9. Unii-usd5x0z99i
10. Nsc-759817
11. 4-oxo-4-(trimethyl[?]yl)oxy-butanoic Acid
12. .beta.-artesunate
13. Artesunate, .beta.-
14. Mls000759445
15. Mls001424053
16. Schembl5694921
17. Chebi:94751
18. Dtxsid30861106
19. Hms2051i14
20. Zinc8214489
21. .beta.-artesunate, (+)-
22. Akos016015102
23. Ccg-100875
24. Nc00125
25. Nsc 759817
26. Ab00698329-05
27. Sr-01000763413-3
28. Sr-01000763413-5
29. Q27166540
30. 4-oxo-4-(((3r,5as,6r,8as,9r,10r,12r,12ar)-3,6,9-trimethyldecahydro-3h-3,12-epoxy[1,2]dioxepino[4,3-i]isochromen-10-yl)oxy)butanoic Acid
31. 4-oxo-4-(((3r,5as,6r,8as,9r,10r,12r,12ar)-3,6,9-trimethyldecahydro-3h-3,12-epoxy[1,2]dioxepino[4,3-i]isochromen-10-yl)oxy)butanoicacid
32. 4-oxo-4-(((3r,5as,6r,8as,9r,10s,12r,12ar)-3,6,9-trimethyldecahydro-3,12-epoxypyrano(4,3-j)-1,2-benzodioxepin-10-yl Hydrogen Butanedioate
33. Butanedioic Acid, Mono((3r,5as,6r,8as,9r,10s,12r,12ar)-decahydro-3,6,9-trimethyl-3,12-epoxy-12h-pyrano(4,3-j)-1,2-benzodioxepin-10-yl) Ester
34. Butanedioic Acid, 1-((3r,5as,6r,8as,9r,10r,12r,12ar)-decahydro-3,6,9-trimethyl-3,12-epoxy-12h-pyrano(4,3-j)-1,2-benzodioxepin-10-yl) Ester
| Molecular Weight | 384.4 g/mol |
|---|---|
| Molecular Formula | C19H28O8 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 384.17841785 g/mol |
| Monoisotopic Mass | 384.17841785 g/mol |
| Topological Polar Surface Area | 101 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 623 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Therap Cat: Antimalarial
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 140
Artesunate Rectal Capsules is indicated for the initial management of acute malaria in patients who cannot take medication by mouth and for whom parenteral treatment is not available.
FDA; FDA Briefing Document for the Anti-Infective Drug Products Advisory Committee. Artesunate Rectal Capsules World Health Organization NDA 21-242. July 10, 2002. U.S. Food and Drug Administration, Center for Drug Evaluation and Research, Division of Special Pathogen and Immunologic Drug Products, 37 pp
To counter the threat of resistance of P. falciparum to monotherapies, and to improve treatment outcome, combinations of antimalarials are now recommended by WHO for the treatment of falciparum malaria. The following ACTs are currently recommended (alphabetical order): AS+AQ artesunate + amodiaquine combination, AS+MQ artesunate + mefloquine combination, AS+SP artesunate + sulfadoxine-pyrimethamine combination.
WHO; WHO Guidelines for the Treatment of Malaria (2006). Available from, as of July 31, 2006: https://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
Artemisinin and its derivatives (artesunate, artemether, artemotil, dihydroartemisinin) produce rapid clearance of parasitaemia and rapid resolution of symptoms. They reduce parasite numbers by a factor of approximately 10,000 in each asexual cycle, which is more than other current antimalarials (which reduce parasite numbers 100- to 1000-fold per cycle). Artemisinin and its derivatives are eliminated rapidly. When given in combination with rapidly eliminated compounds (tetracyclines, clindamycin), a 7-day course of treatment with an artemisinin compound is required; but when given in combination with slowly eliminated antimalarials, shorter courses of treatment (3 days) are effective. The evidence of their superiority in comparison to monotherapies has been clearly documented.
WHO; WHO Guidelines for the Treatment of Malaria (2006). Available from, as of July 31, 2006: https://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
For more Therapeutic Uses (Complete) data for ARTESUNIC ACID (14 total), please visit the HSDB record page.
Artemisinin congeners should not be given to patients with a previous history of an allergic reaction following their consumption or if an urticarial rash develops during treatment. Patient with a history of hypersensitivity reaction to one of the artemsinins should be advised not to take any of the derivatives again.
WHO; Artesunate Rectal Capsules (2002). FDA Division of Anti-Infective Drug Products Advisory Committee Briefing Document. The World Health Organization, Geneva, Switzerland, 53 pp
Artesunate rectal capsules have not been evaluated as sole therapy for malaria; consequently all patient who are initially treated with artesunate rectal capsules should be promptly referred and evaluated at the nearest health care facility able to provide a full curative course of treatment for malaria.
FDA; FDA Briefing Document for the Anti-Infective Drug Products Advisory Committee. Artesunate Rectal Capsules World Health Organization NDA 21-242. July 10, 2002. U.S. Food and Drug Administration, Center for Drug Evaluation and Research, Division of Special Pathogen and Immunologic Drug Products, 37 pp
Adverse events described /following artesunate/ included bitter taste, mild pain at the injection site, bradycardia, paroxysmal ventricular premature beat, incomplete right bundle branch block, first-degree atrio-ventricular block, and urticaria.
FDA; FDA Briefing Document for the Anti-Infective Drug Products Advisory Committee. Artesunate Rectal Capsules World Health Organization NDA 21-242. July 10, 2002. U.S. Food and Drug Administration, Center for Drug Evaluation and Research, Division of Special Pathogen and Immunologic Drug Products, 37 pp
... The most commonly reported adverse events (in the order of <1%) to be mild gastrointestinal (nausea, vomiting, diarrhea, abdominal pain) events.
FDA; FDA Briefing Document for the Anti-Infective Drug Products Advisory Committee. Artesunate Rectal Capsules World Health Organization NDA 21-242. July 10, 2002. U.S. Food and Drug Administration, Center for Drug Evaluation and Research, Division of Special Pathogen and Immunologic Drug Products, 37 pp
For more Drug Warnings (Complete) data for ARTESUNIC ACID (25 total), please visit the HSDB record page.
Treatment of severe malaria caused by Plasmodium falciparum
Treatment of malaria
Artesunate Amivas is indicated for the initial treatment of severe malaria in adults and children.
Consideration should be given to official guidance on the appropriate use of antimalarial agents.
P01BE03
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01B - Antimalarials
P01BE - Artemisinin and derivatives, plain
P01BE03 - Artesunate
Following administration to humans, artesunate is rapidly hydrolyzed to its principal active metabolite, dihydroartemisinin. The pharmacokinetics of artesunate are characterized by marked inter-subject variability, differing significantly between healthy volunteers and infected patients, and among patients with different disease severity.
WHO; Artesunate Rectal Capsules (2002). FDA Division of Anti-Infective Drug Products Advisory Committee Briefing Document. The World Health Organization, Geneva, Switzerland, 53 pp
The pharmacokinetic of artesunate and dihydroartemisin are characterized by marked inter-subject variability. The pharmacokinetic parameters of artesunate and dihydroartemisinin differ significantly between healthy volunteers and infected patients, and among patients with different disease severity. Pharmacokinetic data from unbound plasma concentrations of artesunate or dihydroartemisinin should be interpreted with caution because the drug accumulates selectively in parasitized RBC's In in vitro experiments, accumulation of dihydroartemisinin in infected RBC's is in concentrations approximately 300-fold higher than those in plasma .
WHO; Artesunate Rectal Capsules (2002). FDA Division of Anti-Infective Drug Products Advisory Committee Briefing Document. The World Health Organization, Geneva, Switzerland, 53 pp
The pharmacokinetics of oral dihydroartemisinin (DHA) following the dose of 2 and 4 mg/ kg body weight dihydroartemisinin and 4 mg/kg body weight oral artesunate (AS) were investigated in 20 healthy Thai volunteers (10 males, 10 females). All formulations were generally well tolerated. Oral DHA was rapidly absorbed from gastrointestinal tract with marked inter-individual variation. The pharmacokinetics of DHA following the two dose levels were similar and linearity in its kinetics was observed. Based on the model-independent pharmacokinetic analysis, median (95% CI) values for Cmax of 181 (120-306) and 360 (181-658) ng/ml were achieved at 1.5 hours following 2 and 4 mg/kg body weight dose, respectively. The corresponding values for AUC0-infinity, t1/2z, CL/f and Vz/f were 377 (199-1,128) vs 907 (324-2,289) ng.hr/mL, 0.96 (0.70-1.81) vs 1.2 (0.75-1.44) hours, 7.7 (4.3-12.3) vs 6.6 (3.1-10.1) L/kg, and 90.5 (28.6-178.2) vs 6.6 (3.1-10.1) mL/min/kg, respectively (2 vs 4 mg/kg dose). Oral AS was rapidly biotransformed to DHA, which was detectable in plasma as early as 15 minutes of AS dosing. Following 4 mg/kg dose, median (95% CI) value for Cmax of 519 (236-284) ng/mL was achieved at 0.7 (0.25-1.5) hours. AUC0-infinity, and t1/2z were 657 (362-2,079) ng.hr/mL, 0.74 (0.34-1.42) hours, respectively. Cmax of DHA following oral AS were significantly higher, but total systemic exposure was greater following oral DHA at the same dose level (4 mg/kg body weight). There was no significant sex difference in pharmacokinetics of DHA
PMID:15689069 Na-Bangchang K et al; Southeast Asian J Trop Med Public Health 35 (3): 575-82 (2004)
The aims of this study were to determine the pharmacokinetic parameters of a single dose of 200 mg oral and rectal artesunate in healthy volunteers, and to suggest a rational dosage regimen for rectal administration. The study design was a randomized open cross-over study of 12 healthy volunteers... Pharmacokinetic parameters were derived from the main metabolite alpha-dihydroartemisinin data due to the rapid disappearance of artesunate from the plasma. Dihydroartemisinin following oral administration of artesunate had a significantly higher AUC(0-infinity) (P<0.05 95% confidence interval (CI) -1168.73, -667.61 ng x hr/mL(-1)) and Cmax (P<0.05; 95% CI -419.73, -171.44 ng/mL(-1)), and had shorter tmax (P<0.05; 95% CI -0.97, -0.10 hr) than that following rectal artesunate. There was no statistically significant difference in the elimination half-life between both routes of administration (P>0.05; 95% CI -0.14, 0.53 hr). The relative bioavailability of rectal artesunate was [mean (coefficient of variation %) 54.9 (24.8%) %].
PMID:15267037 Awad MI et al; Trop Doct 34 (3): 132-5 (2004)
For more Absorption, Distribution and Excretion (Complete) data for ARTESUNIC ACID (8 total), please visit the HSDB record page.
Following administration to humans, artesunate is rapidly hydrolyzed to its principle active metabolite, dihydroartemisinin. Data from in vitro studies with human liver microsomes and from clinical studies suggest that DHA-glucoronide (10-position) is the principal Phase II metabolite of DHA and that uridine diphosphate glucuronyl transferase isoforms 1A1, 1A8-9, or 2B7 may be the main conjugating enzyme.
WHO; Artesunate Rectal Capsules (2002). FDA Division of Anti-Infective Drug Products Advisory Committee Briefing Document. The World Health Organization, Geneva, Switzerland, 53 pp
Artemisinin is completely and rapidly absorbed after oral administration in rats. However, a very low plasma level was obtained even after a dose of 300 mg/kg. Liver was found to be the chief site of inactivation. When artemisinin was given i.m., significant and more persistent plasma levels were detected. Artemisinin was shown to pass the blood-brain and blood-placenta barriers after i.v. injection. Very little unchanged artemisinin was found in the urine or feces in 48 hours regardless of the route of administration. Metabolites identified after administration to humans include deoxyartemisinin, deoxydihydroartemisinin, and 9,10-dihydroxydeoxyartemisinin.
PMID:2084705 Lee IS, Hufford CD; Pharmacol Ther. 48 (3): 345-355 (1990)
In volunteer studies, artsunate was cleared very rapidly (within minutes) by biotransformation to dihydroartemisinin, which was eliminated by with a half-life of approximately 45 minutes.
PMID:8153997 White N; Trans. R. Soc. Trop. Med. Hyg 88 (suppl. 1): 41-43 (1994)
Two theories have been put forward for the mode of antimalarial action of the artemisinin antimalarials, in accodance with the known properties of peroxides with medicinal activity. The first assumes that the artemisinins must be activated by contact with either reduced haem (ferrous haem, Fe(ll)PPIX) or non-haem ferrous iron (exogenous iron), causing cleavage of the peroxide to generate oxygen-centered radicals (alkoxy radicals') which are the presumed to be converted into carbon-centered radicals by transfer of proximate hydrogen atoms from the periphery of the peroxide molecule. These carbon-centered radicals are then thought to alkylate sensitive, yet unspecified, biomolecules in the parasite. A second theory argues for a process in which the intact artemisinin binds to a site within a vital protein in the parasite. The act of binding causes the peroxide to be converted to hydroperoxide or similar open peroxide, which in accordance with known properties of such compounds, generates one or more active chemical entities, either oxidizing agents or oxygen transfer agents per se, or oxygen-centered free radicals. This would be associated with the binding process. In such a way, the artemisinins might act as (irreversibile) inhibitors. Iron may, or may not, be associated with the activation process. No specific biological target in the parasite has yet been identified in support of this theory, but it may be membrane-bound proteins.
WHO; Artesunate Rectal Capsules (2002). FDA Division of Anti-Infective Drug Products Advisory Committee Briefing Document. The World Health Organization, Geneva, Switzerland, 53 pp
Artesunate is a water soluble derivative of artemisinin, an antimalarial compound isolated from the Chinese herb Qinghao (Artemisia annua). Artesunate is rapidly metabolized to dihydroartemisinin (DHA) in the body. Chemically, artesunate, and its active metabolite, DHA, are sesquiterpene lactones with a trioxane ring containing a peroxide bridge. The peroxide bridge appears to be essential for the antimalarial activity of artesunate. Structure-activity relationship studies show that the deoxy derivative of DHA (that lack the peroxide bridge) was 277-fold less active than DHA. The activity of deoxyartesunate was not measured. Deoxy derivatives of other artemisinin analogs were 10- to 1000-fold less active compared to the parent compounds. Artesunate increases superoxide anion production and lipid peroxidation in falciparum-infected erythrocytes in vitro. However, artesunate does not suppress the activity of antioxidant enzymes (superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase) in infected or uninfected erythrocytes. Erythrocytes infected with the ring or trophozoite forms in vitro accumulate 100- and 180- fold higher concentrations of DHA (12 nM ie, 3.40 ng/mL), respectively, compared to uninfected erythrocytes. These experiments were performed in a medium containing 10% human serum. The relevance of these findings to the uptake in vivo is unclear. The precise mechanism by which artesunate exhibits antiplasmodial activity is not understood.
FDA; FDA Briefing Document for the Anti-Infective Drug Products Advisory Committee. Artesunate Rectal Capsules World Health Organization NDA 21-242. July 10, 2002. U.S. Food and Drug Administration, Center for Drug Evaluation and Research, Division of Special Pathogen and Immunologic Drug Products, 37 pp