
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
API
0
Data Compilation #PharmaFlow
0
Weekly News Recap #Phispers

1. Nubeqa
2. Odm-201
3. Orm-16497
4. Orm-16555
1. Odm-201
2. 1297538-32-9
3. Bay-1841788
4. Nubeqa
5. Bay1841788
6. Darolutamide [usan]
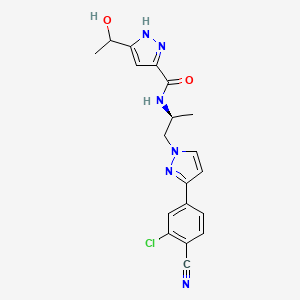
7. N-((s)-1-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl)propan-2-yl)-5-(1-hydroxyethyl)-1h-pyrazole-3-carboxamide
8. Bay 1841788
9. X05u0n2rco
10. Odm-201;bay-1841788
11. 1h-pyrazole-3-carboxamide, N-((1s)-2-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl)-1-methylethyl)-5-(1-hydroxyethyl)-
12. N-((s)-1-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl)-propan-2-yl)-3-(1-hydroxyethyl)-1h-pyrazole-5-carboxamide
13. Odm201
14. 1h-pyrazole-3-carboxamide, N-[(1s)-2-[3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl]-1-methylethyl]-5-(1-hydroxyethyl)-
15. Nebeqa (tn)
16. Darolutamide [mi]
17. Odm-201(darolutamide)
18. Darolutamide [inn]
19. Darolutamide [jan]
20. Unii-x05u0n2rco
21. Darolutamide (odm-201)
22. Darolutamide [who-dd]
23. Darolutamide (jan/usan/inn)
24. Schembl1814935
25. Chembl4297185
26. Schembl13733117
27. Gtpl10439
28. Ex-a759
29. Bdbm309979
30. Darolutamide [orange Book]
31. Dtxsid101027953
32. Example 56 [us9657003]
33. N-[(2s)-1-[3-(3-chloro-4-cyanophenyl)pyrazol-1-yl]propan-2-yl]-5-(1-hydroxyethyl)-1h-pyrazole-3-carboxamide
34. Bdbm50556205
35. Mfcd29472270
36. Nsc825331
37. Akos030526387
38. Ccg-268640
39. Cs-5174
40. Db12941
41. Nsc-825331
42. Ncgc00484078-01
43. Ac-32628
44. As-75032
45. Bay-1841788)
46. Hy-16985
47. S7559
48. J3.501.129c
49. D11045
50. Us9657003, 56
51. A888821
52. J-690121
53. Q25091391
54. 1-[(2s)-2-butanyl]-n-[(4,6-dimethyl-2-oxo-1,2-dihydro-3-pyridinyl)methyl]-3-methyl-6-[6-(1-piperazinyl)-3-pyridinyl]-1h-indole-4-car Boxamide
55. N-((2s)-1-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol- 1-yl)propan-2-yl)-5-((1rs)-1-hydroxyethyl)-1h-pyrazole- 3-carboxamide
56. N-((2s)-1-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl)propan-2-yl)-5-((1rs)-1-hydroxyethyl)-1h-pyrazole-3-carboxamide
57. N-((s)-1-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol -1-yl)propan-2-yl)-3-(1-hydroxyethyl)-1h-pyrazole-5-carboxamide
58. N-((s)-1-(3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl)propan-2-yl)-5-(1-hydroxy-ethyl)-1h-pyrazole-3-carboxamide
59. N-[(2s)-1-[3-(3-chloro-4-cyanophenyl)-1h-pyrazol-1-yl]propan-2-yl]-5-(1-hydroxyethyl)-1h-pyrazole-3-carboxamide
| Molecular Weight | 398.8 g/mol |
|---|---|
| Molecular Formula | C19H19ClN6O2 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 398.1258016 g/mol |
| Monoisotopic Mass | 398.1258016 g/mol |
| Topological Polar Surface Area | 120 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 598 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
This drug is indicated for the treatment of patients diagnosed with non-metastatic and castrate-resistant prostate cancer.
FDA Label
Nubeqa is indicated for the treatment of adult men with non metastatic castration resistant prostate cancer (nmCRPC) who are at risk of developing metastatic disease.
Darolutamide, through its downstream effects on cancer cell growth, treats castrate-resistant prostate cancer. It inhibits cancer cell growth and markedly lowers prostate specific antigen (PSA) levels through potent androgen receptor antagonism.
L02BB
L - Antineoplastic and immunomodulating agents
L02 - Endocrine therapy
L02B - Hormone antagonists and related agents
L02BB - Anti-androgens
L02BB06 - Darolutamide
Absorption
Darolutamide is absorbed in the gastrointestinal tract. In the fasted state, peak concentrations are reached within 3-5 hours, and within 3-8 hours in the fed state. Median Tmax is between 3-6 hours.The average darolutamide steady-state peak plasma concentration after a 600 mg twice daily dose is approximately 4.79 mg/L. The Cmax is attained approximately 4 hours after administration of a single 600 mg oral dose. The AUC 0-12h is approximately 52.82 hg/mL. **Effects of food** The absolute bioavailability of darolutamide is approximately 30% after fasting and taking a single 300 mg dose. Steady-state concentrations are attained between 2 and 5 days after repeated administration with food. The bioavailability of darolutamide increases by 2.0 to 2.5 times when it is given with food.
Route of Elimination
In a pharmacokinetic study, a radiolabeled dose of darolutamide in an oral solution showed that 63.4% of darolutamide-related material was excreted in the urine (7% of which was unchanged drug) and 32.4% in the feces (with 30% unchanged drug).
Volume of Distribution
After intravenous administration, the apparent volume of distribution of darolutamide is about 119L.
Clearance
The clearance of darolutamide after an intravenous dose is 116 mL/min (39.7%).
Darolutamide is mainly metabolized by the CYP3A4 hepatic microsomal enzyme in addition to UGT1A9 and UGT1A1. The main active metabolite keto-darolutamide in found in the plasma at 2 times the concentration of darolutamide.
The half-life of darolutamide and its active metabolite, keto-darolutamide is about 20 hours. A phase 1 study determined a terminal half life ranging between 10-15 hours.
The actions of androgens on androgen receptors (AR) potentiate the growth and survival of prostate cancer cells. Darolutamide competitively inhibits androgens from binding to their receptors, inhibiting AR nuclear translocation, as well as AR-mediated transcription. The end result of these processes is a decrease in prostate cancer cell proliferation and tumor size. Its main metabolite, keto-darolutamide, shows similar pharmacological activity to the parent drug, darolutamide. Darolutamide has been found to bind more tightly to the AR receptor than [apalutamide] and [enzalutamide], which are other androgen receptor antagonists. Darolutamide can act as a progesterone receptor (PR) antagonist in the laboratory setting with approximately 1% activity when compared to its actions at the androgen receptor. The clinical relevance is not known at this time.
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.

Registrant Name : Bayer Korea Ltd.
Registration Date : 2020-05-27
Registration Number : Su212-7-ND
Manufacturer Name : Fermion Oy
Manufacturer Address : Orioninkatu 2, 10960 Hanko Pohjoinen Finland
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
 Lee Fine Chem: Advancing Innovation and Quality in Intermediates, APIs, and Dosage Forms for Superior Pharmaceutical Solutions.
Lee Fine Chem: Advancing Innovation and Quality in Intermediates, APIs, and Dosage Forms for Superior Pharmaceutical Solutions.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 42127
Submission : 2025-06-30
Status : Active
Type : II



GDUFA
DMF Review : Reviewed
Rev. Date : 2023-04-20
Pay. Date : 2023-03-30
DMF Number : 37912
Submission : 2023-03-30
Status : Active
Type : II



GDUFA
DMF Review : Reviewed
Rev. Date : 2023-06-13
Pay. Date : 2023-04-25
DMF Number : 38274
Submission : 2023-05-02
Status : Active
Type : II

NDC Package Code : 68554-0140
Start Marketing Date : 2019-07-30
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36697
Submission : 2022-02-28
Status : Active
Type : II

Date of Issue : 2025-09-19
Valid Till : 2028-05-05
Written Confirmation Number : WC-0349
Address of the Firm :

NDC Package Code : 54893-0107
Start Marketing Date : 2021-01-22
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
About the Company : Biophore, founded in 2007, develops and manufactures niche and complex pharmaceutical products. With USFDA- and EU-approved API facilities, a dedicated intermediates site and an R&...
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
About the Company : Founded in 1984, DRL is well-known for its generic APIs and its track record in drug product development. It is one of the earliest pharma API manufacturers with a diverse portfoli...
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
About the Company : Founded in 1935, TAPI Technology & API Services has a long legacy of advancing health through innovation. Today, we offer one of the industry’s most comprehensive API portfolios ...
 Lee Fine Chem: Advancing Innovation and Quality in Intermediates, APIs, and Dosage Forms for Superior Pharmaceutical Solutions.
Lee Fine Chem: Advancing Innovation and Quality in Intermediates, APIs, and Dosage Forms for Superior Pharmaceutical Solutions.
About the Company : Lee Fine Chem Private Limited specializes in advanced intermediates, Active Pharmaceutical Ingredients (APIs), and finished dosage forms. Focused on quality and innovation, we deli...
About the Company : Sichuan Qingmu Pharmaceutical is an innovation-driven company specializing in generic APIs, advanced intermediates, and CDMO/CMO services for small-molecule drugs. Approved by US F...
About the Company : Alembic Pharmaceuticals Limited is a leading pharmaceutical company in India. The Company is vertically integrated with the ability to develop, manufacture and market pharmaceutica...

About the Company : Fujian Fukang Pharmaceutical Co., Ltd (Fukang) is located in Fuzhou-capital of Fujian province, founded in 1958, renamed Fuzhou Antibiotic General Factory in 1992 and became a whol...

About the Company : Globe Quimica S.A. is a major Brazilian API producer, GMP certificated by ANVISA, manufactures more than 20 different API's such as Antiretrovirals, Anxiolytic, Antidepressant, Ant...

About the Company : Jiangsu Lianhuan Pharmaceutical Co., Ltd. was founded in 2000. It is a state-controlled competitive listed company. Its predecessor was Yangzhou Pharmaceutical Factory, which was e...

About the Company : Consistent growth and sustainability is a multidimensional aspiration for all at Macleods, we remained focused on providing quality and affordable medicines to billions of ailing p...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
84
PharmaCompass offers a list of Darolutamide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Darolutamide manufacturer or Darolutamide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Darolutamide manufacturer or Darolutamide supplier.
PharmaCompass also assists you with knowing the Darolutamide API Price utilized in the formulation of products. Darolutamide API Price is not always fixed or binding as the Darolutamide Price is obtained through a variety of data sources. The Darolutamide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Darolutamide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Darolutamide, including repackagers and relabelers. The FDA regulates Darolutamide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Darolutamide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Darolutamide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Darolutamide supplier is an individual or a company that provides Darolutamide active pharmaceutical ingredient (API) or Darolutamide finished formulations upon request. The Darolutamide suppliers may include Darolutamide API manufacturers, exporters, distributors and traders.
click here to find a list of Darolutamide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Darolutamide DMF (Drug Master File) is a document detailing the whole manufacturing process of Darolutamide active pharmaceutical ingredient (API) in detail. Different forms of Darolutamide DMFs exist exist since differing nations have different regulations, such as Darolutamide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Darolutamide DMF submitted to regulatory agencies in the US is known as a USDMF. Darolutamide USDMF includes data on Darolutamide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Darolutamide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Darolutamide suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Darolutamide Drug Master File in Korea (Darolutamide KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Darolutamide. The MFDS reviews the Darolutamide KDMF as part of the drug registration process and uses the information provided in the Darolutamide KDMF to evaluate the safety and efficacy of the drug.
After submitting a Darolutamide KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Darolutamide API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Darolutamide suppliers with KDMF on PharmaCompass.
A Darolutamide written confirmation (Darolutamide WC) is an official document issued by a regulatory agency to a Darolutamide manufacturer, verifying that the manufacturing facility of a Darolutamide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Darolutamide APIs or Darolutamide finished pharmaceutical products to another nation, regulatory agencies frequently require a Darolutamide WC (written confirmation) as part of the regulatory process.
click here to find a list of Darolutamide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Darolutamide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Darolutamide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Darolutamide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Darolutamide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Darolutamide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Darolutamide suppliers with NDC on PharmaCompass.
Darolutamide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Darolutamide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Darolutamide GMP manufacturer or Darolutamide GMP API supplier for your needs.
A Darolutamide CoA (Certificate of Analysis) is a formal document that attests to Darolutamide's compliance with Darolutamide specifications and serves as a tool for batch-level quality control.
Darolutamide CoA mostly includes findings from lab analyses of a specific batch. For each Darolutamide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Darolutamide may be tested according to a variety of international standards, such as European Pharmacopoeia (Darolutamide EP), Darolutamide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Darolutamide USP).

