Aspen Pharmacare Holdings Ltd
Aspen Pharmacare Holdings Ltd
29 Dec 2025
// PRESS RELEASE
13 Nov 2025
// PRESS RELEASE
13 Nov 2025
// PRESS RELEASE

About
Industry Trade Show
Attending
23-26 March, 2026
Industry Trade Show
Attending
21-23 April, 2026
Industry Trade Show
Booth #814 Level 2
11-14 May, 2026
CONTACT DETAILS





Events
Webinars & Exhibitions
Industry Trade Show
Attending
23-26 March, 2026
Industry Trade Show
Attending
21-23 April, 2026
Industry Trade Show
Booth #814 Level 2
11-14 May, 2026
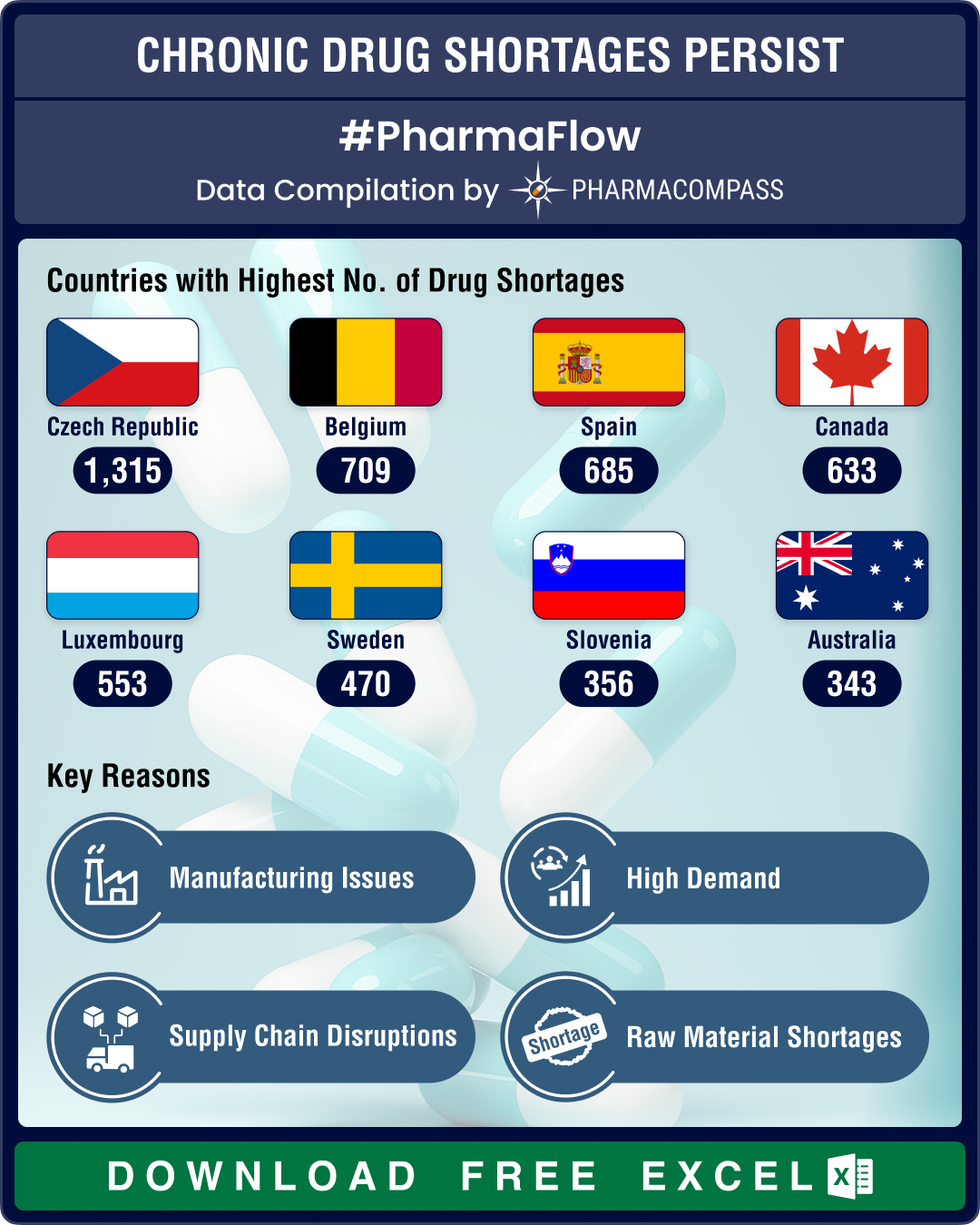
https://www.pharmacompass.com/radio-compass-blog/us-drug-shortages-reduce-16-yoy-in-q1-2025-cns-drugs-antimicrobials-face-highest-scarcities

29 Dec 2025
// PRESS RELEASE
https://www.aspenpharma.com/proposed-divestment-of-aspen-asia-pacific-excluding-china-for-r26-5-billion/

13 Nov 2025
// PRESS RELEASE

13 Nov 2025
// PRESS RELEASE

13 Oct 2025
// REUTERS
https://www.reuters.com/sustainability/boards-policy-regulation/aspen-wins-approval-market-lillys-mounjaro-weight-loss-south-africa-2025-10-13/

04 Sep 2025
// REUTERS
https://www.reuters.com/business/healthcare-pharmaceuticals/south-africas-aspen-aims-profit-turnaround-with-insulin-glp-1-drugs-2025-09-03/

03 Sep 2025
// PRESS RELEASE
https://www.aspenpharma.com/aspen-building-momentum-in-commercial-pharmaceuticals-and-manufacturing-strategy-modified/
ABOUT THIS PAGE