
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
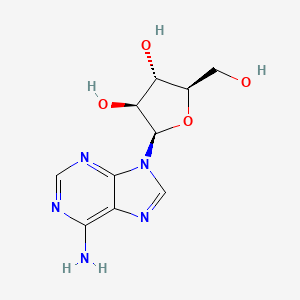

1. 9 Beta Arabinofuranosyladenine
2. 9 Beta D Arabinofuranosyladenine
3. 9-beta-arabinofuranosyladenine
4. 9-beta-d-arabinofuranosyladenine
5. Adenine Arabinoside
6. Alpha Ara A
7. Alpha D Arabinofuranosyladenine
8. Alpha-ara A
9. Alpha-d-arabinofuranosyladenine
10. Ara A
11. Ara-a
12. Arabinofuranosyladenine
13. Arabinoside, Adenine
14. Arabinosyladenine
15. Beta Ara A
16. Beta-ara A
17. Vira A
18. Vira-a
19. Viraa
1. 5536-17-4
2. Adenine Arabinoside
3. Ara-a
4. Vira-a
5. Arabinosyladenine
6. Spongoadenosine
7. Araadenosine
8. Arasena-a
9. 9-beta-d-arabinofuranosyladenine
10. Vidarabine Anhydrous
11. Arabinosyl Adenine
12. Arabinoside Adenine
13. Vidarabin
14. (2r,3s,4s,5r)-2-(6-amino-9h-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol
15. Vidarabine [inn]
16. 9-beta-d-arabinofuranosyl-9h-purin-6-amine
17. 9beta-d-arabinofuranosyladenine
18. Nsc-404241
19. Araa
20. 9-beta-d-arabinofuranosyl-adenine
21. Adenine 9-beta-d-arabinofuranoside
22. Vira Atm
23. 6-amino-9-b-d-arabinofuranosylpurine
24. Vidarabine, Anhydrous
25. Vidarabine (vira-a)
26. Adenine 9-beta;-d-arabinofuranoside
27. Vidarabine (jan)
28. Ci-673
29. 2-(6-amino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol
30. 3xqd2mew34
31. Chembl1090
32. Adenine-beta-d-arabinofuranoside
33. Chebi:45327
34. 9-(beta-d-arabinofuranosyl)adenine
35. 9h-purin-6-amine, 9-.beta.-d-arabinofuranosyl-
36. (2r,3s,4s,5r)-2-(6-amino-9h-purin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol
37. Nsc-247519
38. Nsc-757383
39. Vidarabina [dcit]
40. Vidarabinum
41. Vidarabina
42. Ci 673
43. Rab
44. 9-beta-arabinoadenosine
45. Beta-d-arabinosyladenine
46. Vidarabinum [inn-latin]
47. Vira A
48. Smr000471872
49. Beta-d-arabinofuranosyladenine
50. Ccris 3383
51. Armes (tn)
52. Adenine Beta-d-arabinofuranoside
53. Hsdb 6514
54. Adenine-9-beta-d-arabinofuranoside
55. Adenine, 9beta-d-arabinofuranosyl-
56. Einecs 226-893-9
57. 9h-purin-6-amine, 9-beta-d-arabinofuranosyl-
58. Adenine, 9-beta-d-arabinofuranosyl-
59. Unii-3xqd2mew34
60. Nsc 247519
61. Brn 0624881
62. 6-amino-9-beta-d-arabinofuranosylpurine
63. Ai3-52821
64. 9h-purin-6-amine, 9beta-d-arabinofuranosyl-
65. 9-.beta.-d-arabinofuranosyl-9h-purin-6-amine
66. Spectrum_001894
67. Vidarabine [mi]
68. Spectrum2_001336
69. Spectrum3_000580
70. Spectrum4_000782
71. Spectrum5_001429
72. Vidarabine [hsdb]
73. (2r,3s,4s,5r)-2-(6-aminopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol
74. Vidarabine [who-dd]
75. 9-b-d-arabinofuranosyladenine
76. Bspbio_002000
77. Kbiogr_001224
78. Kbioss_002424
79. Mls001066352
80. Mls001333133
81. Mls001333134
82. Divk1c_000191
83. Schembl110914
84. Spectrum1500609
85. Spbio_001491
86. 9-ss-d-arabinofuranosyladenine
87. Gtpl4806
88. 9-ss -d-arabinofuranosyladenine
89. Hms500j13
90. Kbio1_000191
91. Kbio2_002418
92. Kbio2_004986
93. Kbio2_007554
94. Kbio3_001500
95. Dtxsid80873976
96. 9-beta-d-arabinofuranosyl Adenine
97. Ninds_000191
98. Adenine 9-ss -d-arabinofuranoside
99. Hms1921k05
100. Hms2090f06
101. Hms2092c16
102. Hms2230n24
103. Hms3259i08
104. Hms3714i18
105. Pharmakon1600-01500609
106. Zinc970363
107. Bcp27778
108. Hy-b0277
109. Bdbm50144936
110. Ccg-39634
111. Hg1001
112. Mfcd00065471
113. Nsc757383
114. Pdsp1_001036
115. Pdsp2_001020
116. S1784
117. Akos015919711
118. Db00194
119. Ds-1755
120. Nc00547
121. Idi1_000191
122. Smp1_000312
123. 9-(beta-d-arabinofuranosyl)-9h-adenine
124. 9--d-arabinofuranosyl-9h-purin-6-amine
125. 9h-adenin-9-yl Beta-d-arabinofuranoside
126. Ncgc00023673-08
127. Ncgc00178869-01
128. 6-amino-9-(beta-d-arabinofuranosyl)purine
129. Sbi-0051555.p002
130. Adenine 9-beta-d-arabinofuranoside, >=99%
131. Sw199234-2
132. V0098
133. V0175
134. D06298
135. 9-.beta.-d-arabinofuranosyl-9h-purine-6-amine
136. Ab00430250-08
137. Ab00430250-09
138. Ab00430250_11
139. Ab00430250_12
140. 536v174
141. Q415107
142. Sr-01000765435
143. Sr-05000001737
144. Sr-01000765435-2
145. Sr-05000001737-1
146. Vidarabine, United States Pharmacopeia (usp) Reference Standard
147. (2r,3s,4s,5r)-2-(6-aminopurin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol
| Molecular Weight | 267.24 g/mol |
|---|---|
| Molecular Formula | C10H13N5O4 |
| XLogP3 | -1.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 267.09675391 g/mol |
| Monoisotopic Mass | 267.09675391 g/mol |
| Topological Polar Surface Area | 140 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 335 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
A nucleoside antibiotic isolated from Streptomyces antibioticus. It has some antineoplastic properties and has broad spectrum activity against DNA viruses in cell cultures and significant antiviral activity against infections caused by a variety of viruses such as the herpes viruses, the VACCINIA VIRUS and varicella zoster virus
National Library of Medicine - Medical Subject Headings (2007)
Vidarabine has been shown to possess antiviral activity against the following viruses in vitro: Herpes simplex types 1 and 2; vaccinia, varicella-zoster. Except for rhabdovirus and oncornavirus, vidarabine does not display in vitro antiviral activity against other RNA or DNA viruses, including adenovirus.
FDA; Medwatch. Summary of Safety-Related Drug Labeling Changes Approved by FDA. Vira0A added 10/10/97. Available at www.fda.gov/medwatch/safety/1997/aug97.htm as of March 16, 2007
/EXPTL THER/ When adenovirus causes hemorrhagic cystitis in immunocompromised patients, vidarabine is used for its treatment because therapeutic choice is limited. Although vidarabine has been reported to be effective for these patients, its therapeutic basis has not yet been established. Vidarabine dose-dependently inhibited viral replication as assessed by a yield reduction assay. Viral protein synthesis was dose-dependently inhibited by vidarabine but not at all by acyclovir, and the degree of inhibition by vidarabine was different for each of the viral proteins, ranging from 0-40% of the untreated control. These results indicated the specificity and mechanism of action of vidarabine against adenovirus. The concentration of vidarabine and its metabolite in the bladder is suggested to exhibit effective anti-adenoviral activity in suppressing the replication of adenovirus. Thus, /the authors conclude that their/ results support vidarabine therapy as a possible candidate for adenovirus-induced hemorrhagic cystitis in immunocompromised patients.
PMID:15535050 Kurosaki K et al; Antivir Chem Chemother 15 (5): 281-5 (2004) .
/EXPTL THER/ In the present study, effectiveness of topical vidarabine or subsequent 5-fluorouracil (5-FU) administration was examined against persistent genital human papillomavirus (HPV) infection after local surgery. Thirty patients underwent local eradication treatment of uterine cervical intra-epithelial neoplasia (CIN) and stage Ia1 uterine cervical cancers. HPV typing was performed by PCR-RFLP analysis. HPV infection was detected pre-operatively in 29 of 30 patients. Of these, HPV was still present in the 20 patients within two months after the therapy. Topical administration of vidarabine or subsequent 5-FU once a week for four weeks was performed to the post-operative persistent HPV-positive cases. HPV infection was abolished in 1 of 10 (10%) with topical vidarabine, and in 2 of 4 vidarabine-resistant cases (50%) with topical 5-FU. Topical vidarabine or 5-FU treatment is beneficial for HPV-positive cases after local surgical excision.
PMID:12883720 Niwa K et al; Oncol Rep 10 (5): 1437-41 (2003)
For more Therapeutic Uses (Complete) data for VIDARABINE (18 total), please visit the HSDB record page.
Vidarabine has been classified as a potential teratogen and should be used with caution during pregnancy (use only for strong clinical indication in absence of suitable alternative).
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 149
Hallucinosis has been reported with excessive (as opposed to therapeutic) doses /of Vidarabine/.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 666
Vidarabine should be used only under the close supervision of an ophthalmologist.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 1700
Concurrent topical application of vidarabine and a corticosteroid is contraindicated in superficial herpes simplex keratitis. Although concomitant application of vidarabine and a corticosteroid may be of benefit in severe infections, corticosteroids should be used with caution and the patient must be observed closely because of the risk of accelerating the spread of the infection. If a topical corticosteroid is administered concurrently with vidarabine, the possibility of corticosteroid induced adverse ocular effects, including increased intraocular pressure, glaucoma, and cataract formation, must be considered.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 1700
For more Drug Warnings (Complete) data for VIDARABINE (10 total), please visit the HSDB record page.
For treatment of chickenpox - varicella, herpes zoster and herpes simplex
Vidarabine is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and varicella-zoster virus (VZV). The inhibitory activity of Vidarabine is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts Vidarabine into Vidarabine monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In vitro, Vidarabine triphosphate stops the DNA replication of herpes virus by being incorporated into the DNA strand and preventing the formation of phosphodiester bridges between bases. This ultimately leads to destabilization of the viral DNA strands.
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AB - Nucleosides and nucleotides excl. reverse transcriptase inhibitors
J05AB03 - Vidarabine
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AD - Antivirals
S01AD06 - Vidarabine
Absorption
Systemetic absorption of vidarabine should not be expected to occur following ocular administration and swallowing lacrimal secretions.
Vira-A is rapidly deaminated to arabinosylhypoxanthine (Ara-Hx), the principal metabolite. ...Because of the low solubility of Vira-A, trace amounts of both Vira-A and Ara-Hx can be detected in the aqueous humor only if there is an epithelial defect in the cornea. If the cornea is normal, only trace amounts of Ara-Hx can be recovered from the aqueous humor. Systemic absorption of Vira-A should not be expected to occur following ocular administration and swallowing lacrimal secretions.
FDA; Medwatch. Summary of Safety-Related Drug Labeling Changes Approved by FDA. Vira0A added 10/10/97. Available at www.fda.gov/medwatch/safety/1997/aug97.htm as of March 16, 2007
Vidarabine is poorly absorbed following oral, im, or SC administration. Following iv administration of vidarabine, 75-87% of the dose is rapidly deaminated by adenosine deaminase to ara-hypoxanthine. Ara-hypoxanthine also possesses antiviral activity but substantially less than that of vidarabine. Following slow iv administration of vidarabine 10 mg/kg in adults, peak plasma concentrations of the drug range from 0.2-0.4 ug/mL and peak plasma concentrations of ara-hypoxanthine range from 3-6 ug/mL. Plasma concentrations of vidarabine and ara-hypoxanthine are higher and more prolonged in patients with renal impairment.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 404
Vidarabine and ara-hypoxanthine are widely distributed into body tissues and fluids and readily cross the blood-brain barrier. In patients with normal meninges, ara-hypoxanthine concentrations in the CSF are about 33-35% of concurrent plasma concentrations. Vidarabine crosses the placenta in animals. It is not known if vidarabine is distributed into milk. ... Vidarabine is 20-30% bound and ara-hypoxanthine is 0-3% bound to plasma proteins.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 404
Vidarabine and ara-hypoxanthine are excreted mainly by the kidneys. Within 24 hours following iv administration of vidarabine 15 mg/kg in patients with normal renal function, 1-3% of the dose is excreted in urine as vidarabine and 41-53% of the dose is excreted as ara-hypoxanthine. There is no evidence of fecal excretion of the drug or metabolite.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 404
In laboratory animals, vidarabine is rapidly deaminated in the gastrointestinal tract to Ara-Hx.
In laboratory animals, Vira-A is rapidly deaminated in the gastrointestinal tract to arabinosylhypoxanthine (Ara-Hx).
FDA; Medwatch. Summary of Safety-Related Drug Labeling Changes Approved by FDA. Vira0A added 10/10/97. Available at www.fda.gov/medwatch/safety/1997/aug97.htm as of March 16, 2007
Vidarabine is rapidly deaminated, possibly within the cornea, by adenosine deaminase to ara-hypoxanthine. Ara-hypoxanthine also possesses antiviral activity but substantially less than that of vidarabine.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 1700
The plasma half-life of vidarabine in adults with normal renal function is 1.5 hr, and the plasma half-life of ara-hypoxanthine is 3.3 hr.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 404
Vidarabine stops replication of herpes viral DNA in 2 ways: 1) competitive inhibition of viral DNA polymerase, and consequently 2) incorporation into and termination of the growing viral DNA chain. Vidarabine is sequentially phosphorylated by kinases to the triphosphate ara-ATP, which is the active form of vidarabine that acts as both an inhibitor and a substrate of viral DNA polymerase. By acting as a substrate for viral DNA polymerase, ara-ATP competitively inhibits dATP leading to the formation of faulty DNA. Ara-ATP can also be incorporated into the DNA strand to replace many of the adenosine bases, resulting in the disruption of DNA synthesis.
The antiviral mechanism of action has not been established. Vidarabine appears to interfere with the early steps of viral DNA synthesis.
FDA; Medwatch. Summary of Safety-Related Drug Labeling Changes Approved by FDA. Vira-A added 10/10/97. Available at www.fda.gov/medwatch/safety/1997/aug97.htm as of March 16, 2007
The antiviral mechanism of vidarabine is incompletely understood, but vidarabine is an inhibitor of viral DNA synthesis. Cellular enzymes phosphorylate vidarabine to the triphosphate, which inhibits viral DNA polymerase activity in a manner that is competitive with deoxyadenosine triphosphate. Vidarabine triphosphate is incorporated into both cellular and viral DNA, where it may act as a chain terminator. Vidarabine triphosphate also inhibits ribonucleoside reductase, RNA polyadenylation, and S-adenosylhomocysteine hydrolase (SAHH), an enzyme involved in transmethylation reactions.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1328

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
ABOUT THIS PAGE
48
PharmaCompass offers a list of Vidarabine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vidarabine manufacturer or Vidarabine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vidarabine manufacturer or Vidarabine supplier.
PharmaCompass also assists you with knowing the Vidarabine API Price utilized in the formulation of products. Vidarabine API Price is not always fixed or binding as the Vidarabine Price is obtained through a variety of data sources. The Vidarabine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Vidarabine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Vidarabine, including repackagers and relabelers. The FDA regulates Vidarabine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Vidarabine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Vidarabine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Vidarabine supplier is an individual or a company that provides Vidarabine active pharmaceutical ingredient (API) or Vidarabine finished formulations upon request. The Vidarabine suppliers may include Vidarabine API manufacturers, exporters, distributors and traders.
click here to find a list of Vidarabine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Vidarabine DMF (Drug Master File) is a document detailing the whole manufacturing process of Vidarabine active pharmaceutical ingredient (API) in detail. Different forms of Vidarabine DMFs exist exist since differing nations have different regulations, such as Vidarabine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Vidarabine DMF submitted to regulatory agencies in the US is known as a USDMF. Vidarabine USDMF includes data on Vidarabine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Vidarabine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Vidarabine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Vidarabine Drug Master File in Japan (Vidarabine JDMF) empowers Vidarabine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Vidarabine JDMF during the approval evaluation for pharmaceutical products. At the time of Vidarabine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Vidarabine suppliers with JDMF on PharmaCompass.
Vidarabine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Vidarabine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Vidarabine GMP manufacturer or Vidarabine GMP API supplier for your needs.
A Vidarabine CoA (Certificate of Analysis) is a formal document that attests to Vidarabine's compliance with Vidarabine specifications and serves as a tool for batch-level quality control.
Vidarabine CoA mostly includes findings from lab analyses of a specific batch. For each Vidarabine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Vidarabine may be tested according to a variety of international standards, such as European Pharmacopoeia (Vidarabine EP), Vidarabine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Vidarabine USP).

