
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Canada
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
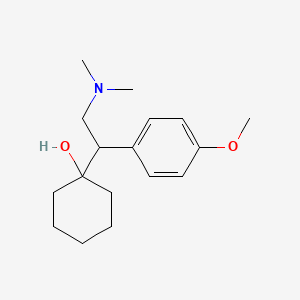

1. 1-(2-(dimethylamino)-1-(4-methoxyphenyl)ethyl)cyclohexanol Hcl
2. Cyclohexanol, 1-(2-(dimethylamino)-1-(4-methoxyphenyl)ethyl)-, Hydrochloride
3. Dobupal
4. Efexor
5. Effexor
6. Hydrochloride, Venlafaxine
7. Sila Venlafaxine
8. Sila-venlafaxine
9. Trevilor
10. Vandral
11. Venlafaxine Hydrochloride
12. Wy 45,030
13. Wy 45030
14. Wy-45,030
15. Wy-45030
16. Wy45,030
17. Wy45030
1. 93413-69-5
2. D,l-venlafaxine
3. Elafax
4. Venlafaxina
5. 1-(2-(dimethylamino)-1-(4-methoxyphenyl)ethyl)cyclohexanol
6. Venlafaxinum
7. Efectin
8. Effexor
9. Velafax
10. Trevilor
11. 1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexanol
12. 1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexan-1-ol
13. Kanghong
14. Venlor
15. Venlafaxine (inn)
16. Grz5rcb1qg
17. Nsc-758676
18. Chebi:9943
19. Cyclohexanol, 1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]-
20. Venlafaxine [inn:ban]
21. Ncgc00095109-01
22. Venlafaxinum [inn-latin]
23. Venlafaxin
24. Venlafaxina [inn-spanish]
25. Venlafaxine [inn]
26. Dsstox_cid_3737
27. Dsstox_rid_77175
28. Dsstox_gsid_23737
29. Venlafexine
30. Efectin (tn)
31. Cas-93413-69-5
32. Sr-01000762930
33. Unii-grz5rcb1qg
34. 1-(2-(dimethylamino)-1-(4-methoxyphenyl)ethyl)cyclohexan-1-ol
35. Dl-venlafaxine
36. Venflaxine Ep
37. Cyclohexanol, 1-(2-(dimethylamino)-1-(4-methoxyphenyl)ethyl)-
38. Hsdb 6699
39. Venlafaxine-d6 Hcl
40. Spectrum_001671
41. Specplus_000842
42. Venlafaxine [mi]
43. Spectrum2_000542
44. Spectrum3_000989
45. Spectrum4_001115
46. Spectrum5_001516
47. Venlafaxine Ep Impurity H
48. Chembl637
49. Ec 618-944-2
50. Venlafaxine [vandf]
51. Schembl35154
52. Bspbio_002657
53. Kbiogr_001590
54. Kbioss_002151
55. Venlafaxine [who-dd]
56. Mls006011859
57. Divk1c_006938
58. Spectrum1504171
59. Spbio_000583
60. Gtpl7321
61. Dtxsid6023737
62. Bdbm82071
63. Kbio1_001882
64. Kbio2_002151
65. Kbio2_004719
66. Kbio2_007287
67. Kbio3_001877
68. Hms1922f05
69. Hms2090f04
70. Hms2093a12
71. Hms3714k11
72. Hms3886k14
73. Pharmakon1600-01504171
74. 1-{2-(dimethylamino)-1-[4-(methyloxy)phenyl]ethyl}cyclohexanol
75. Amy39116
76. Hy-b0196
77. Tox21_111425
78. Ccg-39577
79. Mfcd00864385
80. N,n-dimethyl-2-(1-hydroxycyclohexyl)-2-(4-methoxyphenyl)ethylamine
81. Nsc_62923
82. Nsc758676
83. S5655
84. Stk621394
85. Akos005555049
86. Cyclohexanol, 1-(2-(dimethylamino)-1-(4-methoxyphenyl)ethyl)-, (+-)-
87. Tox21_111425_1
88. Wy 45030wy 45030
89. Ac-1547
90. Db00285
91. Nsc 758676
92. Sdccgsbi-0052740.p003
93. Ncgc00095109-02
94. Ncgc00095109-03
95. Ncgc00095109-04
96. Ncgc00095109-05
97. Ncgc00095109-06
98. Ncgc00095109-08
99. Ncgc00095109-20
100. As-76254
101. Smr002204138
102. Wy-45655
103. Sbi-0052740.p002
104. Cas_93413-69-5
105. Cas_99300-78-4
106. Db-057399
107. Ft-0642242
108. Ft-0675793
109. Ft-0675795
110. C07187
111. D08670
112. D82446
113. Ab00053751-08
114. Ab00053751-10
115. Ab00053751-11
116. Ab00053751_12
117. Ab00053751_13
118. 413v695
119. Q898407
120. Sr-01000762930-3
121. Sr-01000762930-4
122. Brd-a51714012-001-02-3
123. Brd-a51714012-003-01-1
124. 1-[2-dimethylamino-1-(4-methoxylphenyl)ethyl]cyclohexanol
125. (rs)-1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexanol
126. (+/-)-1-(.alpha.-((dimethylamino)methyl)-p-methoxybenzyl)cyclohexanol
127. 540726-98-5
| Molecular Weight | 277.4 g/mol |
|---|---|
| Molecular Formula | C17H27NO2 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 277.204179104 g/mol |
| Monoisotopic Mass | 277.204179104 g/mol |
| Topological Polar Surface Area | 32.7 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 279 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antidepressive Agents, Second-Generation; Serotonin Uptake Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 2011)
Venlafaxine hydrochloride is used in the treatment of major depressive disorder. /Included in US product labeling/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2360
Venlafaxine hydrochloride is used in the treatment of generalized anxiety disorder. /Included in US product labeling/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2360
Venlafaxine hydrochloride is used in the treatment of social phobia (social anxiety disorder). /Included in US product labeling/
2011 Emergency Response Planning Guidelines (ERPG) & Workplace Exposure Level (WEEL). American Industrial Hygiene Association, Fairfax, VA 2011, p. 2360
For more Therapeutic Uses (Complete) data for Venlafaxine (10 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: SUICIDAL THOUGHTS AND BEHAVIORS. Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term studies. These studies did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in patients over age 24; there was a reduction in risk with antidepressant use in patients aged 65 and older. In patients of all ages who are started on antidepressant therapy monitor closely for clinical worsening and emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber.
US Natl Inst Health; DailyMed. Current Medication Information for EFFEXOR (venlafaxine hydrochloride) capsule, extended release (Updated: February 2015). Available from, as of April 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=53c3e7ac-1852-4d70-d2b6-4fca819acf26
The US Food and Drug Administration (FDA) recommends that all patients being treated with antidepressants for any indication be appropriately monitored and closely observed for clinical worsening, suicidality, and unusual changes in behavior, particularly during initiation of therapy (i.e., the first few months) and during periods of dosage adjustments. Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be advised to monitor patients on a daily basis for the emergence of agitation, irritability, or unusual changes in behavior, as well as the emergence of suicidality, and to report such symptoms immediately to a health-care provider.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2361
Although a causal relationship between the emergence of symptoms such as anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia, hypomania, and/or mania and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality. Consequently, consideration should be given to changing the therapeutic regimen or discontinuing therapy in patients whose depression is persistently worse or in patients experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, particularly if such manifestations are severe, abrupt in onset, or were not part of the patient's presenting symptoms. If a decision is made to discontinue therapy, venlafaxine dosage should be tapered as rapidly as is feasible but with recognition of the risks of abrupt discontinuance.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2361
A case of venlafaxine-induced serotonin syndrome is described with relapse following the introduction of amitriptyline, despite a 2-week period between the discontinuation of one drug and the commencement of the other. Electroencephalography may play an important part in diagnosis. With the increasing use of selective serotonin re-uptake inhibitors, greater awareness of the serotonin syndrome is necessary. Furthermore, the potential for drug interactions which may lead to the syndrome needs to be recognized.
Perry N; Postgrad Med J 76(894): p.254-256 (2000)
For more Drug Warnings (Complete) data for Venlafaxine (20 total), please visit the HSDB record page.
Venlafaxine is indicated in the management of major depressive disorder (MDD), generalized anxiety disorder (GAD), social anxiety disorder (social phobia), and panic disorder with or without agoraphobia. Venlafaxine is also used off-label for prophylaxis of migraine headaches, for reduction of vasomotor symptoms associated with menopause, and for management of neuropathic pain (although there is only minimal evidence of efficacy for this condition). It is also considered a second-line option for management of obsessive-compulsive disorder (OCD).
The mechanism of venlafaxine's (and its metabolite, O-desmethylvenlafaxine (ODV)) antidepressant effect is believed to be due to their potentiation of neurotransmitter activity in the central nervous system through the inhibition of the reuptake of serotonin and norepinephrine from within the synapse. Venlafaxine has also been shown to weakly inhibit dopamine reuptake. Neither venlafaxine nor ODV bind to muscarinic, histaminergic, or alpha-1 adrenergic receptors; pharmacologic activity at these receptors is hypothesized to be associated with the various anticholinergic, sedative, and cardiovascular effects seen with other psychotropic drugs. Hyponatremia has also been shown to occur as a result of treatment with SNRIs, and is associated with the development of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Venlafaxine also demonstrates a clinically significant and dose-related effect on blood pressure, likely due to its potentiation of norepinephrine.
Antidepressive Agents, Second-Generation
A structurally and mechanistically diverse group of drugs that are not tricyclics or monoamine oxidase inhibitors. The most clinically important appear to act selectively on serotonergic systems, especially by inhibiting serotonin reuptake. (See all compounds classified as Antidepressive Agents, Second-Generation.)
Serotonin and Noradrenaline Reuptake Inhibitors
Drugs that selectively block or suppress the plasma membrane transport of SEROTONIN and NORADRENALINE into axon terminals and are used as ANTIDEPRESSIVE AGENTS. (See all compounds classified as Serotonin and Noradrenaline Reuptake Inhibitors.)
N06AX16
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N06AX16
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N06AX16
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N06AX16
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N06AX16
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N06AX16
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N06AX16
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AX - Other antidepressants
N06AX16 - Venlafaxine
Absorption
Venlafaxine is well absorbed, with at least 92% of a single dose absorbed on the basis of mass balance studies. Food does not affect the absorption of venlafaxine or its subsequent metabolism into ODV. Bioavailability is 45% following oral administration. Time to steady state = 3 days.
Route of Elimination
Renal elimination of venlafaxine and its metabolites is the primary route of excretion. Approximately 87% of a venlafaxine dose is recovered in the urine within 48 hours as either unchanged venlafaxine (5%), unconjugated ODV (29%), conjugated ODV (26%), or other minor inactive metabolites (27%).
Volume of Distribution
7.5 3.7 L/kg [venlafaxine]
5.7 1.8 L/kg [O-desmethylvenlafaxine(active metabolite)]
Clearance
Steady state plasma clearance, venlafaxine = 1.3 0.6 L/h/kg; Steady state plasma clearance, ODV = 0.4 0.2 L/h/kg.
Venlafaxine is well absorbed ... .On the basis of mass balance studies, at least 92% of a single oral dose of venlafaxine is absorbed. The absolute bioavailability of venlafaxine is about 45%
US Natl Inst Health; DailyMed. Current Medication Information for EFFEXOR (venlafaxine hydrochloride) capsule, extended release (October 2009). Available from, as of November 8, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=venlafaxine
Steady-state concentrations of venlafaxine and O-desmethylvenlafaxine in plasma are attained within 3 days of oral multiple dose therapy. Venlafaxine and O-desmethylvenlafaxine exhibited linear kinetics over the dose range of 75 to 450 mg/day. Mean +/-SD steady-state plasma clearance of venlafaxine and O-desmethylvenlafaxine is 1.3 +/- 0.6 and 0.4 0.2 L/hr/kg, respectively; apparent elimination half-life is 5 +/- 2 and 11 +/- 2 hours, respectively; and apparent (steady-state) volume of distribution is 7.5 +/- 3.7 and 5.7 +/- 1.8 L/kg, respectively. Venlafaxine and O-desmethylvenlafaxine are minimally bound at therapeutic concentrations to plasma proteins (27% and 30%, respectively).
US Natl Inst Health; DailyMed. Current Medication Information for EFFEXOR (venlafaxine hydrochloride) capsule, extended release (October 2009). Available from, as of November 8, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=venlafaxine
Approximately 87% of a venlafaxine dose is recovered in the urine within 48 hours as unchanged venlafaxine (5%), unconjugated O-desmethylvenlafaxine (29%), conjugated O-desmethylvenlafaxine (26%), or other minor inactive metabolites (27%). Renal elimination of venlafaxine and its metabolites is thus the primary route of excretion
US Natl Inst Health; DailyMed. Current Medication Information for EFFEXOR (venlafaxine hydrochloride) capsule, extended release (October 2009). Available from, as of November 8, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=venlafaxine
Venlafaxine is a unique antidepressant ... . The pharmacokinetics and relative bioavailability of venlafaxine were evaluated in healthy volunteers after oral administration. The bioavailability of 50 mg of venlafaxine as a tablet relative to a solution was determined in a two-period randomized crossover study. The rate of absorption from the gastrointestinal tract was assessed by the time to peak plasma concentration (tmax), a model-dependent calculation of the first-order absorption rate constant, and a model-independent calculation of mean residence time. The extent of absorption was assessed by peak plasma concentration (Cmax) and area under the concentration-time curve (AUC). No statistically significant differences were observed between the two formulations for either the rate or extent of absorption. Similarly, systemic concentrations of the active O-demethylated metabolite did not significantly differ after administration of the two venlafaxine formulations. AUC ratios indicated that the relative bioavailabilities of the parent drug, and formulation of metabolite were approximately 98% and 92%, respectively, for the tablet versus the solution. A separate study was conducted to examine the influence of food on venlafaxine absorption from the 50-mg tablet. A standard, medium-fat breakfast eaten immediately before drug administration delayed the tmax of venlafaxine but did not affect Cmax or AUC. Therefore the tablet formulation of venlafaxine is bioequivalent to the oral solution, and the presence of food appears to decrease the rate but not the extent of absorption of venlafaxine from the tablet formulation.
Troy S et al; J Clin Pharmacol 37(10): p.954-961 (1997)
For more Absorption, Distribution and Excretion (Complete) data for Venlafaxine (8 total), please visit the HSDB record page.
Undergoes extensive first pass metabolism in the liver to its major, active metabolite, O-desmethylvenlafaxine ODV, and two minor, less active metabolites, N-desmethylvenlafaxine and N,O-didesmethylvenlafaxine. Formation of ODV is catalyzed by cytochrome P450 (CYP) 2D6, whereas N-demethylation is catalyzed by CYP3A4, 2C19 and 2C9. ODV possesses antidepressant activity that is comparable to that of venlfaxine.
Following absorption, venlafaxine undergoes extensive presystemic metabolism in the liver, primarily to O-desmethylvenlafaxine, but also to N-desmethylvenlafaxine, N,O-didesmethylvenlafaxine, and other minor metabolites. In vitro studies indicate that the formation of O-desmethylvenlafaxine is catalyzed by CYP2D6; this has been confirmed in a clinical study showing that patients with low CYP2D6 levels ("poor metabolizers") had increased levels of venlafaxine and reduced levels of O-desmethylvenlafaxine compared to people with normal CYP2D6 ("extensive metabolizers"). The differences between the CYP2D6 poor and extensive metabolizers, however, are not expected to be clinically important because the sum of venlafaxine and O-desmethylvenlafaxine is similar in the two groups and venlafaxine and O-desmethylvenlafaxine are pharmacologically approximately equiactive and equipotent.
US Natl Inst Health; DailyMed. Current Medication Information for EFFEXOR (venlafaxine hydrochloride) capsule, extended release (October 2009). Available from, as of November 8, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=venlafaxine
The biotransformation of venlafaxine (VF) into its two major metabolites, O-desmethylvenlafaxine (ODV) and N-desmethylvenlafaxine (NDV) was studied in vitro with human liver microsomes and with microsomes containing individual human cytochromes from cDNA-transfected human lymphoblastoid cells. VF was coincubated with selective cytochrome P450 (CYP) inhibitors and several selective serotonin reuptake inhibitors (SSRIs) to assess their inhibitory effect on VF metabolism. Formation rates for ODV incubated with human microsomes were consistent with Michaelis-Menten kinetics for a single-enzyme mediated reaction with substrate inhibition. Mean parameters determined by non-linear regression were: Vmax = 0.36 nmol/min/mg protein, K(m) = 41 microM, and Ks 22901 microM (Ks represents a constant which reflects the degree of substrate inhibition). Quinidine (QUI) was a potent inhibitor of ODV formation with a Ki of 0.04 microM, and paroxetine (PX) was the most potent SSRI at inhibiting ODV formation with a mean Ki value of 0.17 microM. Studies using expressed cytochromes showed that ODV was formed by CYP2C9, -2C19, and -2D6. CYP2D6 was dominant with the lowest K(m), 23.2 microM, and highest intrinsic clearance (Vmax/K(m) ratio). No unique model was applicable to the formation of NDV for all four livers tested. Parameters determined by applying a single-enzyme model were Vmax = 2.14 nmol/min/mg protein, and K(m) = 2504 microM. Ketoconazole was a potent inhibitor of NDV production, although its inhibitory activity was not as great as observed with pure 3A substrates. NDV formation was also reduced by 42% by a polyclonal rabbit antibody against rat liver CYP3A1. Studies using expressed cytochromes showed that NDV was formed by CYP2C9, -2C19, and -3A4. The highest intrinsic clearance was attributable to CYP2C19 and the lowest to CYP3A4. However the high in vivo abundance of 3A isoforms will magnify the importance of this cytochrome. Fluvoxamine (FX), at a concentration of 20 microM, decreased NDV production by 46% consistent with the capacity of FX to inhibit CYP3A, 2C9, and 2C19. These results are consistent with previous studies that show CYP2D6 and -3A4 play important roles in the formation of ODV and NDV, respectively. In addition we have shown that several other CYPs have important roles in the biotransformation of VF.
PMID:10192828 Fogelman S et al; Neuropsychopharmacology 20(5): 480-490 (1999)
On three occasions, unusually high trough plasma concentrations of venlafaxine were measured in a patient phenotyped and genotyped as being an extensive CYP2D6 metabolizer and receiving 450 mg/day of venlafaxine and multiple comedications. Values of 1.54 and of 0.60 mg/l of venlafaxine and O-desmethylvenlafaxine, respectively, were determined in the first blood sample, giving an unusually high venlafaxine to O-desmethylvenlafaxine ratio. This suggests an impaired metabolism of venlafaxine to O-desmethylvenlafaxine, and is most likely due to metabolic interactions with mianserin (240 mg/day) and propranolol (40 mg/day). Concentration of (S)-venlafaxine measured in this blood sample was almost twice as high as (R)-venlafaxine ((S)/(R) ratio: 1.94). At the second blood sampling, after addition of thioridazine (260 mg/day), which is a strong CYP2D6 inhibitor, concentrations of venlafaxine were further increased (2.76 mg/l), and concentrations of O-desmethylvenlafaxine decreased (0.22 mg/l). A decrease of the (S)/(R)-venlafaxine ratio (-20%) suggests a possible stereoselectivity towards the (R)-enantiomer of the enzyme(s) involved in venlafaxine O-demethylation at these high venlafaxine concentrations. At the third blood sampling, after interruption of thioridazine, concentrations of venlafaxine and O-desmethylvenlafaxine were similar to those measured in the first blood sample. This case report shows the importance of performing studies on the effects of either genetically determined or acquired deficiency of metabolism on the kinetics of venlafaxine.
Eap C et al; Pharmacopsychiatry 33(3): p.112-115 (2000)
Approximately 87% of a venlafaxine dose is recovered in the urine within 48 hours as unchanged venlafaxine (5%), unconjugated O-desmethylvenlafaxine (29%), conjugated O-desmethylvenlafaxine (26%), or other minor inactive metabolites (27%). Renal elimination of venlafaxine and its metabolites is thus the primary route of excretion
US Natl Inst Health; DailyMed. Current Medication Information for EFFEXOR (venlafaxine hydrochloride) capsule, extended release (October 2009). Available from, as of November 8, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=venlafaxine
5 hours
Apparent elimination half-life /of venlafaxine and O-desmethylvenlafaxine/ is 5 +/- 2 and 11 +/- 2 hours, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for EFFEXOR (venlafaxine hydrochloride) capsule, extended release (October 2009). Available from, as of November 8, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=venlafaxine
The exact mechanism of action of venlafaxine is unknown, but appears to be associated with the potentiation of neurotransmitter activity in the CNS. Venlafaxine and its active metabolite, O-desmethylvenlafaxine (ODV), inhibit the reuptake of both serotonin and norepinephrine with a potency greater for the 5-HT than for the NE reuptake process. Both venlafaxine and the ODV metabolite have weak inhibitory effects on the reuptake of dopamine but, unlike the tricyclics and similar to SSRIs, they are not active at histaminergic, muscarinic, or alpha(1)-adrenergic receptors.
The mechanism of the antidepressant action of venlafaxine in humans is believed to be associated with its potentiation of neurotransmitter activity in the CNS. Preclinical studies have shown that venlafaxine and its active metabolite, O-desmethylvenlafaxine, are potent inhibitors of neuronal serotonin and norepinephrine reuptake and weak inhibitors of dopamine reuptake. Venlafaxine and O-desmethylvenlafaxine have no significant affinity for muscarinic cholinergic, H1-histaminergic, or a1-adrenergic receptors in vitro. Pharmacologic activity at these receptors is hypothesized to be associated with the various anticholinergic, sedative, and cardiovascular effects seen with other psychotropic drugs. Venlafaxine and O-desmethylvenlafaxine do not possess monoamine oxidase (MAO) inhibitory activity.
US Natl Inst Health; DailyMed. Current Medication Information for EFFEXOR (venlafaxine hydrochloride) capsule, extended release (October 2009). Available from, as of November 8, 2011: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=venlafaxine
/The antinociceptive effects of the novel phentylethylamine antidepressant drug venlafaxine and its interaction with various opioid, noradrenaline and serotonin receptor subtypes were evaluated. When mice were tested with a hotplate analgesia meter, venlafaxine induced a dose-dependent antinociceptive effect following i.p. administration with an ED50 of 46.7 mg/kg (20.5; 146.5; 95% CL). Opioid, adrenergic and serotoninergic receptor antagonists were tested for their ability to block venlafaxine antinociception. Venlafaxine-induced antinociception was significantly inhibited by naloxone, nor-BNI and naltrindole but not by beta-FNA or naloxonazine, implying involvement of kappa1- and delta-opioid mechanisms. When adrenergic and serotoninergic antagonists were used, yohimbine (P < 0.005) but not phentolamine or metergoline, decreased antinociception elicited by venlafaxine, implying a clear alpha2- and a minor alpha1-adrenergic mechanism of antinociception. When venlafaxine was administered together with various agonists of the opioid and alpha2- receptor subtypes, it significantly potentiated antinociception mediated by kappa1- kappa3- and delta-opioid receptor subtypes. The alpha2-adrenergic agonist clonidine significantly potentiated venlafaxine-mediated antinociception. Summing up these results, we conclude that the antinociceptive effect of venlafaxine is mainly influenced by the kappa- and delta-opioid receptor subtypes combined with the alpha2-adrenergic receptor. These results suggest a potential use of venlafaxine in the management of some pain syndromes. However, further research is needed in order to establish both the exact clinical indications and the effective doses of venlafaxine when prescribed for pain. /Salt not specified/
Schreiber S et al; Neurosci Letters 273(2): p.85-88 (1999)
Venlafaxine ... causes selective inhibition of neuronal reuptake of serotonine and norepinephrine with little effect on other neurotransmitter systems. Cases of seizures, tachycardia, and QRS prolongation have been observed following drug overdose in humans. The clinical manifestations of cardiac toxicity suggest that venlafaxine may exhibit cardiac electrophysiological effects on fast conducting cells. Consequently, studies were undertaken to characterize effects of venlafaxine on the fast inward sodium current (I(Na)) of isolated guinea pig ventricular myocytes. Currents were recorded with the whole-cell configuration of the patch-clamp technique in the presence of Ca(2+) and K(+) channel blockers. Results obtained demonstrated that venlafaxine inhibits peak I(Na) in a concentration-dependent manner with an estimated IC(50) of 8. 10(-6) M. Inhibition was exclusively of a tonic nature and rate-independent. Neither kinetics of inactivation (tau(inac)= 0.652 +/- 0.020 ms, under control conditions; tau(inac)= 0.636 +/- 0.050, in the presence of 10(-5) M venlafaxine; n = 5 cells isolated from five animals) nor kinetics of recovery from inactivation of the sodium channels (tau(re)= 58.7 +/- 1.6 ms, under control conditions; tau(re)= 54.4 +/- 1.8, in the presence of 10(-5) M venlafaxine; n = 10 cells isolated from six animals) were significantly altered by 10(-5) M venlafaxine. These observations led us to conclude that venlafaxine blocks I(Na) following its binding to the resting state of the channel. Thus, the characteristics of block of I(Na) by venlafaxine are different from those usually observed with most tricyclic antidepressants or conventional class I antiarrhythmic drugs.
Khalifa M et al; J Pharmacol Exp Ther 291(1): p.280-284 (1999)
The 5-HT(4) receptor may be a target for antidepressant drugs. ... The effects of the dual antidepressant, venlafaxine, on 5-HT(4) receptor-mediated signalling events /is examined/. The effects of 21 days treatment (p.o.) with high (40 mg kg(-1)) and low (10 mg kg(-1)) doses of venlafaxine, were evaluated at different levels of 5-HT(4) receptor-mediated neurotransmission by using in situ hybridization, receptor autoradiography, adenylate cyclase assays and electrophysiological recordings in rat brain. The selective noradrenaline reuptake inhibitor, reboxetine (10 mg kg(-1), 21 days) was also evaluated on 5-HT(4) receptor density. Treatment with a high dose (40 mg kg(-1)) of venlafaxine did not alter 5-HT(4) mRNA expression, but decreased the density of 5-HT(4) receptors in caudate-putamen (% reduction = 26 + or - 6), hippocampus (% reduction = 39 + or -7 and 39 + or - 8 for CA1 and CA3 respectively) and substantia nigra (% reduction = 49 + or - 5). Zacopride-stimulated adenylate cyclase activation was unaltered following low-dose treatment (10 mg kg(-1)) while it was attenuated in rats treated with 40 mg kg(-1) of venlafaxine (% reduction = 51 + or - 2). Furthermore, the amplitude of population spike in pyramidal cells of CA1 of hippocampus induced by zacopride was significantly attenuated in rats receiving either dose of venlafaxine. Chronic reboxetine did not modify 5-HT(4) receptor density. Data indicate a functional desensitization of 5-HT(4) receptors after chronic venlafaxine, similar to that observed after treatment with the classical selective inhibitors of 5-HT reuptake.
PMID:20880406 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2990165 Vidal R et al; Br J Pharmacol 161 (3): 695-706 (2010)
The present study was undertaken to evaluate the potential role of 5-HT1A receptors in the antidepressant-like effect and antinociceptive effect of venlafaxine. With this aim, the effect of either a selective 5-HT1A receptor antagonist (WAY-100635; N-2-[4-(2-methoxyphenyl-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexane carboxamide) or a selective 5-HT1A receptor agonist (8-OH-DPAT; 8-hydroxy-2-(di-n-propylamine) tetralin hydrobromide) was investigated in mice in combination with venlafaxine by means of the forced swimming test, a paradigm aimed at screening potential antidepressants, and the hot-plate test, a phasic pain model. Surprisingly, the results showed that WAY-100635 produced a large decrease in the antidepressant-like effect of venlafaxine, while 8-OH-DPAT rendered effective a non-effective dose of this antidepressant. However, in the hot-plate test WAY-100635 significantly enhanced the antinociceptive effect of venlafaxine, whereas 8-OH-DPAT counteracted its antinociceptive effect. These findings show that 5-HT1A receptors play differing roles in modulating the antidepressant-like and antinociceptive effects of venlafaxine in the models investigated. The results imply that blockade of the 5-HT1A receptors in the forebrain will counteract the favourable (antidepressant-like) effect at raphe nuclei level, and consequently, the overall effect evidenced is an antagonism. This suggests a predominant role of 5-HT1A receptors located in the forebrain area for the antidepressant-like effect. In contrast, the antinociceptive effect of venlafaxine is probably potentiated due to the blockade of somatodendritic 5-HT1A receptors in the same raphe nuclei, facilitating the descending monoaminergic pain control system.
PMID:18405417 Berrocoso E, Mico JA; Int J Neuropsychopharmacol 12 (1): 61-71 (2009)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
19
PharmaCompass offers a list of Venlafaxine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Venlafaxine manufacturer or Venlafaxine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Venlafaxine manufacturer or Venlafaxine supplier.
PharmaCompass also assists you with knowing the Venlafaxine API Price utilized in the formulation of products. Venlafaxine API Price is not always fixed or binding as the Venlafaxine Price is obtained through a variety of data sources. The Venlafaxine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Venlafaxine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Venlafaxine, including repackagers and relabelers. The FDA regulates Venlafaxine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Venlafaxine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Venlafaxine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Venlafaxine supplier is an individual or a company that provides Venlafaxine active pharmaceutical ingredient (API) or Venlafaxine finished formulations upon request. The Venlafaxine suppliers may include Venlafaxine API manufacturers, exporters, distributors and traders.
click here to find a list of Venlafaxine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Venlafaxine DMF (Drug Master File) is a document detailing the whole manufacturing process of Venlafaxine active pharmaceutical ingredient (API) in detail. Different forms of Venlafaxine DMFs exist exist since differing nations have different regulations, such as Venlafaxine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Venlafaxine DMF submitted to regulatory agencies in the US is known as a USDMF. Venlafaxine USDMF includes data on Venlafaxine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Venlafaxine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Venlafaxine suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Venlafaxine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Venlafaxine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Venlafaxine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Venlafaxine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Venlafaxine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Venlafaxine suppliers with NDC on PharmaCompass.
Venlafaxine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Venlafaxine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Venlafaxine GMP manufacturer or Venlafaxine GMP API supplier for your needs.
A Venlafaxine CoA (Certificate of Analysis) is a formal document that attests to Venlafaxine's compliance with Venlafaxine specifications and serves as a tool for batch-level quality control.
Venlafaxine CoA mostly includes findings from lab analyses of a specific batch. For each Venlafaxine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Venlafaxine may be tested according to a variety of international standards, such as European Pharmacopoeia (Venlafaxine EP), Venlafaxine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Venlafaxine USP).

