
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
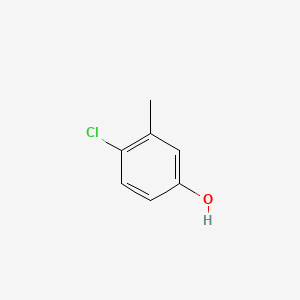

1. 4-chloro-3-cresol
2. 4-chloro-meta-cresol
3. Chlorocresol
4. Chlorocresol Sodium Salt
1. Chlorocresol
2. 59-50-7
3. 4-chloro-m-cresol
4. P-chloro-m-cresol
5. Phenol, 4-chloro-3-methyl-
6. Parol
7. P-chlorocresol
8. 4-chloro-3-cresol
9. Ottafact
10. Baktol
11. Candaseptic
12. 2-chloro-5-hydroxytoluene
13. Baktolan
14. Parmetol
15. Peritonan
16. Raschit
17. Aptal
18. Rasen-anicon
19. 4-chloro-5-methylphenol
20. Pcmc
21. Preventol Cmk
22. Raschit K
23. P-chlor-m-cresol
24. 6-chloro-3-hydroxytoluene
25. 3-methyl-4-chlorophenol
26. 2-chloro-hydroxytoluene
27. Parachlorometacresol
28. Chlorocresolum
29. 4-chloro-3-methyl Phenol
30. M-cresol, 4-chloro-
31. Rcra Waste Number U039
32. Nsc 4166
33. Para-chloro-meta-cresol
34. Chebi:34395
35. Nsc-4166
36. Mfcd00002323
37. 1-chloro-2-methyl-4-hydroxybenzene
38. Ncgc00091338-01
39. Chlorcresolum
40. Chlorkresolum
41. Chlorocresolo
42. Chlorokresolum
43. Perol
44. 36w53o7109
45. Dsstox_cid_1717
46. Chloro-3-cresol
47. Dsstox_rid_76291
48. Dsstox_gsid_21717
49. Clorocresolo [dcit]
50. Clorocresol [spanish]
51. Caswell No. 185a
52. Chlorocresolum [latin]
53. Clorocresol
54. Clorocresolo
55. Clorocresol [inn-spanish]
56. Cas-59-50-7
57. Chlorocresolum [inn-latin]
58. Ccris 1938
59. 4-chloro-meta-cresol
60. Hsdb 5198
61. 4-chloro-1-hydroxy-3-methylbenzene
62. Einecs 200-431-6
63. 4-chloro-3-methyl-phenol
64. Rcra Waste No. U039
65. Epa Pesticide Chemical Code 064206
66. Brn 1237629
67. Lysochlor
68. Chlorocresol [usan:inn:nf]
69. Ai3-00075
70. Chlorocresol, Nf
71. Unii-36w53o7109
72. Spectrum_000130
73. 2p7a
74. 4-chlor-3-methylphenol
75. Chlorocresol (nf/inn)
76. Spectrum2_000002
77. Spectrum4_000278
78. Spectrum5_000705
79. Chlorocresol [ii]
80. Chlorocresol [mi]
81. 4-chloro-5-methyl-phenol
82. Chlorocresol [inn]
83. Wln: Qr Dg C
84. Chlorocresol [hsdb]
85. Chlorocresol [usan]
86. Ec 200-431-6
87. Schembl12344
88. Chlorocresol [mart.]
89. Kbiogr_000776
90. Kbioss_000590
91. Mls002152924
92. Bidd:er0169
93. Chlorocresol [who-dd]
94. Chlorocresol [who-ip]
95. Divk1c_000768
96. Phenol, 4-chloro-5-methyl-
97. Spectrum1500178
98. Spbio_000003
99. Zinc1124
100. Chembl1230222
101. Dtxsid4021717
102. 4-chloro-3-methylphenol, 99%
103. Hms502g10
104. Kbio1_000768
105. Kbio2_000590
106. Kbio2_003158
107. Kbio2_005726
108. Nsc4166
109. Ninds_000768
110. P-chloro-m-cresol [inci]
111. Hms1920o03
112. Hms2091c14
113. Hms3652f13
114. Hms3885p09
115. Pharmakon1600-01500178
116. Chlorocresol [ep Monograph]
117. Hy-b1284
118. Tox21_111116
119. Tox21_201293
120. Tox21_300054
121. Bdbm50527069
122. Ccg-39979
123. Hsci1_000352
124. Nsc756680
125. S4209
126. Stl268900
127. Chlorocresolum [who-ip Latin]
128. Akos000120242
129. Tox21_111116_1
130. Cs-4678
131. Nsc-756680
132. Chlorocresol (4-chloro-3-methylphenol)
133. Idi1_000768
134. Ncgc00091338-02
135. Ncgc00091338-03
136. Ncgc00091338-04
137. Ncgc00091338-06
138. Ncgc00254021-01
139. Ncgc00258845-01
140. 4-chloro-3-methylphenol, Technical Grade
141. Ac-14332
142. Ls-13269
143. Smr001224524
144. Sbi-0051308.p003
145. 4-chloro-3-methylphenol, Analytical Standard
146. B1696
147. Ft-0618220
148. Sw219289-1
149. 4-chloro-3-methylphenol, >=98.0% (hplc)
150. A16444
151. D03468
152. Ab00051939_02
153. Ab00051939_03
154. Q302865
155. Sr-05000002033
156. 4-chloro-3-methylphenol 100 Microg/ml In Methanol
157. Q-200453
158. Sr-05000002033-1
159. Brd-k89056082-001-03-6
160. F0001-1543
161. Z955123738
162. 43m
| Molecular Weight | 142.58 g/mol |
|---|---|
| Molecular Formula | C7H7ClO |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 142.0185425 g/mol |
| Monoisotopic Mass | 142.0185425 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 94.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Fungicides, Industrial
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
MEDICATION (VET): In topical medicaments and in intrauterine lubricants as antiseptic and preservative (0.1-0.2% concentration).
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 104
Chlorocresol and chloracetamide are used in medications, glues, and cosmetics as preservatives.
PMID:7249630 Dooms-Goossens A et al; Contact Dermatitis 7 (1): 51-2 (1981)
Antiseptic (topical).
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 368
/EXPTL THER/...At all concentrations of /4-chloro-m-cresol/ (4-cmc), the increase in baseline force was significantly greater in the /malignant hyperthermia susceptible/ (MHS) group compared to the /malignant hyperthermia negative/ MHN group (P<0.05). Muscle from 15 MHS patients responded to 4-cmc with increasing force at a threshold concentration of 75 umol/L or less, whereas muscle from 23 MH-non-susceptible (MHN) patients had thresholds of 100 umol/L or more. The accuracy of the chlorocresol test was thus 100% (95% confidence limits 90.75-100%) at a threshold of 75 umol/L. Amplitude of contractures at 2 mmol/L caffeine was not different from contractures at 75 umol/L of 4-cmc in either the MHS or the MHN group (P>0.05). In vivo concentrations of chlorocresol from clinical use of insulin and somatropin are estimated to be 20 times less than the threshold concentration and thus these drugs seem safe in MH patients. 4-chloro-m-cresol may be a suitable aid to clarify puzzling results of standard testing of MH susceptibility.
PMID:9311392 Ording H et al; Acta Anaesthesiol Scand 41 (8): 967-72 (1997)
One source has rated p-chloro-m-cresol as very toxic, with a probable lethal dose to humans of 50 to 500 mg/kg.
Sittig, M. Handbook of Toxic and Hazardous Chemicals and Carcinogens, 1985. 2nd ed. Park Ridge, NJ: Noyes Data Corporation, 1985., p. 230
Fungicides, Industrial
Chemicals that kill or inhibit the growth of fungi in agricultural applications, on wood, plastics, or other materials, in swimming pools, etc. (See all compounds classified as Fungicides, Industrial.)
Four groups of conventional female albino guinea pigs, three per group, were used to determine the bioavailability of PCMC. Occlusive patches of 0.2 mL of a 5% PCMC aqueous suspension stabilized with Carbomer 941, a saturated aqueous solution of 0.38% PCMC, 5% PCMC in olive oil/acetone (4:1), or 5% PCMC in propylene glycol were applied for 24 hr. After 96 hr, the animals were killed and the skin at the site of patch testing was removed for analysis (the patches were kept for analysis to determine the amount of PCMC remaining in the patch material). Fractional sampling of the urine and feces was performed to determine the rate of absorption of PCMC. An additional three animals had been injected with PCMC intraperitoneally to determine the excretion rate. However, no free PCMC was found, indicating rapid metabolism. In determining bioavailability, the calculations were based on the assumption that the saturated PCMC solution is 0.4% (w/v), corresponding to 0.8 mg in 0.2 mL, and that 0.2 mL of the 5% PCMC preparations contained 10 mg of the chemical. The results indicated that 25% of the aqueous PCMC (stabilized with carbomer 941) and 46% of the saturated aqueous PCMC solution remained in the patches. Only 0.2% of the aqueous PCMC (stabilized with carbomer 941) and 0.5% of the saturated aqueous PCMC solution was found in the skin at the patch site. This was compared to 65% of the PCMC in propylene glycol and 66% of the PCMC in olive oil/acetone solutions remaining in the patch; and 0.7% and 1.6%, respectively found in the skin at the patch site. The authors conclude that PCMC was more bioavailable from the aqueous preparations. After 96 hr, 0.2 and 0.5% PCMC was detected at the patch test site in the animals dosed with 5% and saturated aqueous PCMC, respectively, and 0.7 and 1.6% PCMC were found in the skin of the animals patch tested with 5% PCMC in olive oil/acetone and propylene glycol, respectively.
Cosmetic Ingredient Expert Review Panel; Final Report on the Safety Assessment for p-Chloro-m-Cresol. International Journal of Toxicology 16 (3): 235-68 (1997). https://esis.jrc.ec.europa.eu/
The biocide 4-chloro-3-methylphenol (CMP, CAS number 59-50-7) is a common additive to metal-working fluids (MWF) and building materials. ... In the current study, the in vitro human epidermal permeability of CMP contained in a working dilution of TRIM VX (20% in water) was evaluated and, for comparison, permeability from an aqueous buffer was also assessed. CMP penetration was also measured from transient exposures to 20% TRIM VX. ... Transient (20 or 40 min) exposures of epidermal membranes to 20% TRIM VX (/sample size/ = 4) resulted in total penetration of 4.2 +/- 1.2 and 7.3 +/- 0.8 ug/sq cm, respectively. ...
PMID:20818538 Frasch HF et al; J Toxicol Environ Health A 73 (20): 1394-405 (2010)
A pharmacokinetic study was performed in which rats were dosed orally with 300 mg/kg PCMC. PCMC reportedly was eliminated rapidly through the kidneys. Additionally, there is no likelihood of cumulation effects. A corresponding examination of fatty and hepatic tissues from rats that were fed 150-1500 ppm PCMC for up to 13 week reported no indication of an accumulation of PCMC in these tissues.
Cosmetic Ingredient Expert Review Panel; Final Report on the Safety Assessment for p-Chloro-m-Cresol. International Journal of Toxicology 16 (3): 235-68 (1997).
Rabbits were injected subcutaneously with 1 g 3-methyl-4-chlorophenol/kg bw dissolved in olive oil. 15-20% of the 3-methyl-4-chlorophenol given was excreted (urine) during 4-5 days.
European Chemicals Bureau; IUCLID Dataset for 3-Methyl-4-Chlorophenol (59-50-7), p.57(2000). Available from, as of July 22, 2011: https://esis.jrc.ec.europa.eu/
For more Absorption, Distribution and Excretion (Complete) data for 3-METHYL-4-CHLOROPHENOL (7 total), please visit the HSDB record page.
...In skeletal muscle sarcoplasmic reticulum, 4-chloro-m-cresol was found to be a potent activator of Ca2+ release mediated by a ruthenium red/caffeine-sensitive Ca2+ release channel. In cerebellar microsomes, this compound released Ca2+ from an inositol-1,4,5-trisphosphate-insensitive store, suggesting that there too it was acting at the ryanodine receptor level. When tested on PC12 cells, chlorocresol released Ca2+ from a caffeine- and thapsigargin-sensitive intracellular store. In addition, the compound was capable of releasing Ca2+ after pretreatment of PC12 cells with bradykinin, suggesting that it acts on a channel contained within an intracellular Ca2+ store that is distinct from that sensitive to inositol-1,4,5-trisphosphate. Structure-activity relationship analyses suggest that the chloro and methyl groups in chlorocresols are important for the activation of the ryanodine receptor Ca2+ release channel.
PMID:8264556 Zorzato F et al; Mol Pharmacol 44 (6): 1192-201 (1993)
The ryanodine receptor type 1 (RyR1) and type 2 (RyR2), but not type 3 (RyR3), are efficiently activated by 4-chloro-m-cresol (4-CmC). /It was/ previously /shown/ that a 173-amino acid segment of RyR1 (residues 4007-4180) is required for channel activation by 4-CmC ... present study... used site-directed mutagenesis to identify individual amino acid(s) within this region that mediate 4-CmC activation. In RyR1, substitution of 11 amino acids conserved between RyR1 and RyR2, but divergent in RyR3, with their RyR3 counterparts reduced 4-CmC sensitivity to the same degree as substitution of the entire 173-amino acid segment. Further analysis of various RyR1 mutants containing successively smaller numbers of these mutations identified 2 amino acid residues (Gln(4020) and Lys(4021)) that, when mutated to their RyR3 counterparts (Leu(3873) and Gln(3874)), abolished 4-CmC activation of RyR1. Mutation of either of these residues alone did not abolish 4-CmC sensitivity, although Q4020L partially reduced 4-CmC-induced Ca /ion/ transients. In addition, mutation of the corresponding residues in RyR3 to their RyR1 counterparts (L3873Q/Q3874K) imparted 4-CmC sensitivity to RyR3. Recordings of single RyR1 channels indicated that 4-CmC applied to either the luminal or cytoplasmic side activated the channel with equal potency. Secondary structure modeling in the vicinity of the Gln(4020)-Lys(4021) dipeptide suggests that the region contains a surface-exposed region adjacent to a hydrophobic segment, indicating that both hydrophilic and hydrophobic regions of RyR1 are necessary for 4-CmC binding to the channel and/or to translate allosteric 4-CmC binding into channel activation.
PMID:16737973 Fessenden JD et al; J Biol Chem 281 (30): 21022-21031 (2006)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
19
PharmaCompass offers a list of Chlorocresol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Chlorocresol manufacturer or Chlorocresol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Chlorocresol manufacturer or Chlorocresol supplier.
PharmaCompass also assists you with knowing the Chlorocresol API Price utilized in the formulation of products. Chlorocresol API Price is not always fixed or binding as the Chlorocresol Price is obtained through a variety of data sources. The Chlorocresol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tox21_300054 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tox21_300054, including repackagers and relabelers. The FDA regulates Tox21_300054 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tox21_300054 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tox21_300054 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tox21_300054 supplier is an individual or a company that provides Tox21_300054 active pharmaceutical ingredient (API) or Tox21_300054 finished formulations upon request. The Tox21_300054 suppliers may include Tox21_300054 API manufacturers, exporters, distributors and traders.
click here to find a list of Tox21_300054 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tox21_300054 written confirmation (Tox21_300054 WC) is an official document issued by a regulatory agency to a Tox21_300054 manufacturer, verifying that the manufacturing facility of a Tox21_300054 active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Tox21_300054 APIs or Tox21_300054 finished pharmaceutical products to another nation, regulatory agencies frequently require a Tox21_300054 WC (written confirmation) as part of the regulatory process.
click here to find a list of Tox21_300054 suppliers with Written Confirmation (WC) on PharmaCompass.
Tox21_300054 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tox21_300054 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tox21_300054 GMP manufacturer or Tox21_300054 GMP API supplier for your needs.
A Tox21_300054 CoA (Certificate of Analysis) is a formal document that attests to Tox21_300054's compliance with Tox21_300054 specifications and serves as a tool for batch-level quality control.
Tox21_300054 CoA mostly includes findings from lab analyses of a specific batch. For each Tox21_300054 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tox21_300054 may be tested according to a variety of international standards, such as European Pharmacopoeia (Tox21_300054 EP), Tox21_300054 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tox21_300054 USP).

