Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
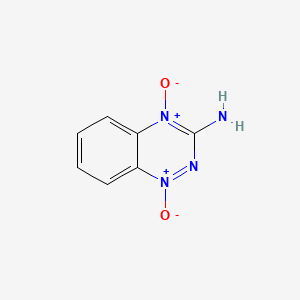

1. 3-amino-1,2,4-benzotriazine-1,4-dioxide
2. Nsc 130181
3. Sr 4233
4. Sr-4233
5. Sr4233
6. Tirazone
7. Win 59075
8. Win-59075
9. Win59075
1. 27314-97-2
2. 3-aminobenzo[e][1,2,4]triazine 1,4-dioxide
3. 1,2,4-benzotriazin-3-amine, 1,4-dioxide
4. Tirazone
5. 3-amino-1,2,4-benzotriazine 1,4-dioxide
6. Win-59075
7. Win 59075
8. Sr 4233
9. Sr-4233
10. Chebi:78887
11. 1,2,4-benzotriazin-3-amine,1,4-dioxide
12. Sr-259075
13. Nsc-130181
14. Sr259075
15. 1ud32yr59g
16. Sr259075;sr4233;win59075
17. Tirapazamine [usan:inn]
18. Nsc 130181
19. Brn 0179322
20. Unii-1ud32yr59g
21. Win59075
22. 1,2,4-benzotriazin-3-amine 1,4-dioxide
23. Sr4233
24. 4-hydroxy-1-oxido-1,2,4-benzotriazin-1-ium-3-imine
25. Tirapazamine [mi]
26. Tirapazamine (usan/inn)
27. Tirapazamine [inn]
28. Tirapazamine [usan]
29. Schembl4048
30. Schembl4049
31. 1,2,4-benzotriazine, 3-amino-, 1,4-dioxide
32. Tirapazamine [mart.]
33. 4-26-00-01120 (beilstein Handbook Reference)
34. Chembl50882
35. Schembl872285
36. Tirapazamine [who-dd]
37. Tirapazamine, >=98% (hplc)
38. Amy38675
39. Bcp03663
40. Ex-a2967
41. Zinc1607808
42. Bdbm50226806
43. Mfcd00132954
44. Akos006271584
45. Akos037645846
46. Db04858
47. Ncgc00390788-04
48. Ac-31305
49. As-64112
50. Hy-13767
51. Bcp0726000167
52. Ft-0661586
53. T3823
54. 3-aminobenzo[e][1,2,4]triazine1,4-dioxide
55. D06167
56. T72208
57. J-016728
58. Q3529346
59. 1,4-dioxido-1,2,4-benzotriazine-1,4-diium-3-amine
60. 3-amino-1,2,4-benzotriazine-1,4-diium-1,4-bis(olate)
61. 4-hydroxy-3-imino-3,4-dihydro-1,2,4-benzotriazin-1-ium-1-olate
| Molecular Weight | 178.15 g/mol |
|---|---|
| Molecular Formula | C7H6N4O2 |
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 178.04907545 g/mol |
| Monoisotopic Mass | 178.04907545 g/mol |
| Topological Polar Surface Area | 89.8 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 191 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of head and neck cancer.
Tirapazamine is a anticancer drug that is inactive in normal tissues that are well oxygenated, but becomes active at the low oxygen levels found in solid tumors. As a result, the drug kills these poorly oxygenated or hypoxic cells while limiting toxicity in normal tissue. Tirapazamine may prove highly effective when used in combination with standard anticancer therapy, as these hypoxic cells are characteristically resistant to radiation and common anticancer agents.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Radiation-Sensitizing Agents
Drugs used to potentiate the effectiveness of radiation therapy in destroying unwanted cells. (See all compounds classified as Radiation-Sensitizing Agents.)
Extensive preclinical testing has established that the mechanism for the selective toxicity towards hypoxic cells is the result of a one-electron reduction of the parent molecule to a free radical species that interacts with DNA to produce single- and double-strand breaks and lethal chromosome aberrations. It has also shown activity when combined with fractionated irradiation and when combined with some chemotherapy agents, particularly cisplatin and carboplatin.
ABOUT THIS PAGE
26
PharmaCompass offers a list of Tirapazamine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tirapazamine manufacturer or Tirapazamine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tirapazamine manufacturer or Tirapazamine supplier.
PharmaCompass also assists you with knowing the Tirapazamine API Price utilized in the formulation of products. Tirapazamine API Price is not always fixed or binding as the Tirapazamine Price is obtained through a variety of data sources. The Tirapazamine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tirapazamine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tirapazamine, including repackagers and relabelers. The FDA regulates Tirapazamine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tirapazamine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Tirapazamine supplier is an individual or a company that provides Tirapazamine active pharmaceutical ingredient (API) or Tirapazamine finished formulations upon request. The Tirapazamine suppliers may include Tirapazamine API manufacturers, exporters, distributors and traders.
Tirapazamine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tirapazamine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tirapazamine GMP manufacturer or Tirapazamine GMP API supplier for your needs.
A Tirapazamine CoA (Certificate of Analysis) is a formal document that attests to Tirapazamine's compliance with Tirapazamine specifications and serves as a tool for batch-level quality control.
Tirapazamine CoA mostly includes findings from lab analyses of a specific batch. For each Tirapazamine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tirapazamine may be tested according to a variety of international standards, such as European Pharmacopoeia (Tirapazamine EP), Tirapazamine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tirapazamine USP).

