
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Agn 190168
2. Agn-190168
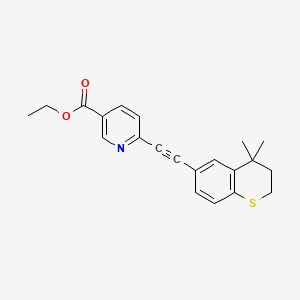
3. Ethyl 6-(2-(4,4-dimethylthiochroman-6-yl)ethynyl)nicotinate
4. Tazorac
1. 118292-40-3
2. Tazorac
3. Zorac
4. Ethyl 6-((4,4-dimethylthiochroman-6-yl)ethynyl)nicotinate
5. Avage
6. Agn-190168
7. Fabior
8. Agn 190168
9. Tazarotene (avage)
10. Idp-123
11. 81bdr9y8ps
12. Chembl1657
13. Ethyl 6-[2-(4,4-dimethyl-2,3-dihydrothiochromen-6-yl)ethynyl]pyridine-3-carboxylate
14. 3-pyridinecarboxylic Acid, 6-((3,4-dihydro-4,4-dimethyl-2h-1-benzothiopyran-6-yl)ethynyl)-, Ethyl Ester
15. Chebi:32184
16. Ethyl 6-[2-(4,4-dimethyl-3,4-dihydro-2h-1-benzothiopyran-6-yl)ethynyl]pyridine-3-carboxylate
17. Ncgc00167525-01
18. Ethyl 6-[(4,4-dimethyl-3,4-dihydro-2h-thiochromen-6-yl)ethynyl]nicotinate
19. Dsstox_cid_26691
20. Dsstox_rid_81825
21. Dsstox_gsid_46691
22. Tazaroteno
23. Tazarotenum
24. Suretin
25. Tazoral
26. Zora
27. 6-(4,4-dimethyl-thiochroman-6-ylethynyl)-nicotinic Acid Ethyl Ester
28. Ethyl 6-((4,4-dimethylthiochroman-6-yl)ethynyl)nicotinate;6-(4,4-dimethyl-thiochroman-6-ylethynyl)-nicotinic Acid Ethyl Ester
29. Tazorac (tn)
30. Avage (tn)
31. Cas-118292-40-3
32. Unii-81bdr9y8ps
33. Acnitaz
34. Ethyl 6-[2-(4,4-dimethylthiochroman-6-yl)ethynyl]pyridine-3-carboxylate
35. Ethyl 6-(2-(4,4-dimethylthiochroman-6-yl)-ethynyl)nicotinate
36. Ethyl 6-[2-(4,4-dimethylthiochroman-6-yl)-ethynyl]nicotinate
37. Tazarotene (jan/usan/inn)
38. Tazarotene [usan:inn:ban]
39. Tazarotene,(s)
40. Tazarotene- Bio-x
41. Fabior (tn)
42. Arazlo
43. Tazarotene [mi]
44. Tazarotene [inn]
45. Tazarotene [jan]
46. Tazarotene [inci]
47. Tazarotene [usan]
48. Tazarotene [vandf]
49. Schembl3134
50. Tazarotene [mart.]
51. Tazarotene [who-dd]
52. Mls003915630
53. Bidd:gt0293
54. Gtpl6952
55. Dtxsid5046691
56. Tazarotene [orange Book]
57. Hms3655k05
58. Hms3747c19
59. Hms3747e19
60. Duobrii Component Tazarotene
61. Act06773
62. Amy31169
63. Bcp22966
64. Zinc1542199
65. Tox21 112522
66. Tox21_112522
67. Ac-755
68. Bdbm50265951
69. Mfcd00867628
70. S1569
71. Idp-118 Component Tazarotene
72. Akos015902872
73. Tazarotene Component Of Duobrii
74. Tox21_112522_1
75. Bs-1012
76. Ccg-268046
77. Cs-1029
78. Db00799
79. Ncgc00167525-02
80. Ncgc00167525-03
81. Bt164442
82. Hy-15388
83. Smr002096194
84. Bcp0726000163
85. Db-014992
86. Ft-0652578
87. Sw220026-1
88. T3108
89. Agn190168, Agn-190168
90. D01132
91. Ab01274801-01
92. Ab01274801_02
93. 292t403
94. A846572
95. Sr-01000931253
96. Q-102516
97. Q3981685
98. Sr-01000931253-2
99. Ethyl 6-[2-(4,4-dimethylthiochroman-6-yl)ethynyl]nicotinate
100. 6-(4,4-dimethylthiochroman-6-ylethynyl) Nicotinic Acid Ethyl Ester
101. (+/-)octahydrocyclopenta[b]pyrrole-2-carboxylicacidhydrochloride
102. 6-(2-(4,4-dimethyl-thiochroman-6yl)ethynyl)-nicotinic Acid Ethyl Ester
103. Ethyl 6-[(4,4-dimethyl-3,4-dihydro-2h-thiochromen-6-yl)ethynyl]pyridine-3-carboxylate
104. 6-[2-(3,4-dihydro-4,4-dimethyl-2h-1-benzothiopyran-6-yl)ethynyl]- 3-pyridinecarboxylic Acid Ethyl Ester
105. 6-[2-(3,4-dihydro-4,4-dimethyl-2h-1-benzothiopyran-6-yl)ethynyl]-3-pyridinecarboxylic Acid Ethyl Ester
106. 6-[2-(3,4-dihydro-4,4-dimethyl-2h-1-benzothiopyran-6-yl)ethynyl]-3-pyridinecarboxylicacidethylester
| Molecular Weight | 351.5 g/mol |
|---|---|
| Molecular Formula | C21H21NO2S |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 351.12930009 g/mol |
| Monoisotopic Mass | 351.12930009 g/mol |
| Topological Polar Surface Area | 64.5 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 547 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Avage |
| PubMed Health | Tazarotene (On the skin) |
| Drug Classes | Dermatological Agent |
| Drug Label | AVAGE Cream is a white cream and contains the compound tazarotene; this formulation of tazarotene cream is also marketed for the treatment of plaque psoriasis and acne vulgaris as TAZORAC (tazarotene) Cream, 0.1%. Tazarotene is a member of the ac... |
| Active Ingredient | Tazarotene |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Allergan |
| 2 of 6 | |
|---|---|
| Drug Name | Fabior |
| PubMed Health | Tazarotene (On the skin) |
| Drug Classes | Dermatological Agent |
| Drug Label | FABIOR (tazarotene) Foam, 0.1% contains the compound tazarotene, a member of the acetylenic class of retinoids. It is for topical use only.Chemically, tazarotene is ethyl 6-[(4,4-dimethylthiochroman-6-yl)ethynyl]nicotinate. The structural formula is... |
| Active Ingredient | Tazarotene |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Stiefel Labs |
| 3 of 6 | |
|---|---|
| Drug Name | Tazorac |
| Drug Label | TAZORAC Gel is a translucent, aqueous gel and contains the compound tazarotene, a member of the acetylenic class of retinoids. It is for topical dermatologic use only. The active ingredient is represented by the following structural formula: Formul... |
| Active Ingredient | Tazarotene |
| Dosage Form | Cream; Gel |
| Route | Topical |
| Strength | 0.05%; 0.1% |
| Market Status | Prescription |
| Company | Allergan |
| 4 of 6 | |
|---|---|
| Drug Name | Avage |
| PubMed Health | Tazarotene (On the skin) |
| Drug Classes | Dermatological Agent |
| Drug Label | AVAGE Cream is a white cream and contains the compound tazarotene; this formulation of tazarotene cream is also marketed for the treatment of plaque psoriasis and acne vulgaris as TAZORAC (tazarotene) Cream, 0.1%. Tazarotene is a member of the ac... |
| Active Ingredient | Tazarotene |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Allergan |
| 5 of 6 | |
|---|---|
| Drug Name | Fabior |
| PubMed Health | Tazarotene (On the skin) |
| Drug Classes | Dermatological Agent |
| Drug Label | FABIOR (tazarotene) Foam, 0.1% contains the compound tazarotene, a member of the acetylenic class of retinoids. It is for topical use only.Chemically, tazarotene is ethyl 6-[(4,4-dimethylthiochroman-6-yl)ethynyl]nicotinate. The structural formula is... |
| Active Ingredient | Tazarotene |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Stiefel Labs |
| 6 of 6 | |
|---|---|
| Drug Name | Tazorac |
| Drug Label | TAZORAC Gel is a translucent, aqueous gel and contains the compound tazarotene, a member of the acetylenic class of retinoids. It is for topical dermatologic use only. The active ingredient is represented by the following structural formula: Formul... |
| Active Ingredient | Tazarotene |
| Dosage Form | Cream; Gel |
| Route | Topical |
| Strength | 0.05%; 0.1% |
| Market Status | Prescription |
| Company | Allergan |
Used to treat psoriasis, acne and sun damaged skin (photodamage).
FDA Label
Treatment of lamellar ichthyosis
Following topical application, tazarotene undergoes esterase hydrolysis to form its active metabolite, tazarotenic acid. When treating acne tazarotene may be taken in conjunction with an oral antibiotic. Tazarotene has been shown in peer-reviewed double blinded studies to reduce: mottling and hyperpigmentation, sallowness, fine wrinkling and coarse wrinkling in sun damaged skin. Histological studies have shown that long term (greater than 1 year) use of Tazarotene is associated with a significant reduction in atypical melanocytes and keratocytes - cells considered to be precursors of skin cancer. Some studies have shown long term use of Tazarotene to be associated with increased collagen production and better organization of skin collagen bundles.
Keratolytic Agents
Agents that soften, separate, and cause desquamation of the cornified epithelium or horny layer of skin. They are used to expose mycelia of infecting fungi or to treat corns, warts, and certain other skin diseases. (See all compounds classified as Keratolytic Agents.)
Teratogens
An agent that causes the production of physical defects in the developing embryo. (See all compounds classified as Teratogens.)
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
D - Dermatologicals
D05 - Antipsoriatics
D05A - Antipsoriatics for topical use
D05AX - Other antipsoriatics for topical use
D05AX05 - Tazarotene
Absorption
Minimal systemic absorption of tazarotene occurs due to its rapid metabolism in the skin to the active metabolite, tazarotenic acid, which can be systemically absorbed and further metabolized. Gender had no influence on the systemic bioavailability of tazarotenic acid.
Route of Elimination
Tazarotene and tazarotenic acid were metabolized to sulfoxides, sulfones and other polar metabolites which were eliminated through urinary and fecal pathways.
Undergoes esterase hydrolysis in skin to form its active metabolite, tazarotenic acid. Tazarotenic acid is further metabolized in skin and, after systemic absorption, hepatically metabolized to sulfoxides, sulfones, and other polar products for elimination.
The half-life of the active form of the drug, tazarotenic acid, is approximately 18 hours in normal and psoriatic patients.
Although the exact mechanism of tazarotene action is not known, studies have shown that the active form of the drug (tazarotenic acid) binds to all three members of the retinoic acid receptor (RAR) family: RARa, RARb, and RARg, but shows relative selectivity for RARb, and RARg and may modify gene expression. It also has affinity for RXR receptors.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
40
PharmaCompass offers a list of Tazarotene API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tazarotene manufacturer or Tazarotene supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tazarotene manufacturer or Tazarotene supplier.
PharmaCompass also assists you with knowing the Tazarotene API Price utilized in the formulation of products. Tazarotene API Price is not always fixed or binding as the Tazarotene Price is obtained through a variety of data sources. The Tazarotene Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tazarotene manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tazarotene, including repackagers and relabelers. The FDA regulates Tazarotene manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tazarotene API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tazarotene manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tazarotene supplier is an individual or a company that provides Tazarotene active pharmaceutical ingredient (API) or Tazarotene finished formulations upon request. The Tazarotene suppliers may include Tazarotene API manufacturers, exporters, distributors and traders.
click here to find a list of Tazarotene suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tazarotene DMF (Drug Master File) is a document detailing the whole manufacturing process of Tazarotene active pharmaceutical ingredient (API) in detail. Different forms of Tazarotene DMFs exist exist since differing nations have different regulations, such as Tazarotene USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tazarotene DMF submitted to regulatory agencies in the US is known as a USDMF. Tazarotene USDMF includes data on Tazarotene's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tazarotene USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tazarotene suppliers with USDMF on PharmaCompass.
A Tazarotene written confirmation (Tazarotene WC) is an official document issued by a regulatory agency to a Tazarotene manufacturer, verifying that the manufacturing facility of a Tazarotene active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Tazarotene APIs or Tazarotene finished pharmaceutical products to another nation, regulatory agencies frequently require a Tazarotene WC (written confirmation) as part of the regulatory process.
click here to find a list of Tazarotene suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Tazarotene as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Tazarotene API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Tazarotene as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Tazarotene and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Tazarotene NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Tazarotene suppliers with NDC on PharmaCompass.
Tazarotene Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tazarotene GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tazarotene GMP manufacturer or Tazarotene GMP API supplier for your needs.
A Tazarotene CoA (Certificate of Analysis) is a formal document that attests to Tazarotene's compliance with Tazarotene specifications and serves as a tool for batch-level quality control.
Tazarotene CoA mostly includes findings from lab analyses of a specific batch. For each Tazarotene CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tazarotene may be tested according to a variety of international standards, such as European Pharmacopoeia (Tazarotene EP), Tazarotene JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tazarotene USP).

