



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
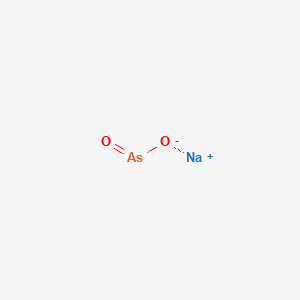

1. Kml001
2. Sodium Meta-arsenite
3. Sodium Metaarsenite
1. 7784-46-5
2. Sodium Metaarsenite
3. Sodium Dioxoarsenate
4. Sodium;oxoarsinite
5. Sodium Meta-arsenite
6. Sodium (meta)arsenite
7. Arsenite, Sodium
8. Naaso2
9. Arsenious Acid, Sodium Salt
10. Arsenious Acid, Monosodium Salt
11. Sodium Arsenenite
12. 48ovy2oc72
13. Chebi:29678
14. Arsenenous Acid, Sodium Salt (1:1)
15. Prodalumnol
16. Sodanit
17. Penite
18. Sodium Arsenite, 0.1n Standardized Solution
19. Kill-all
20. Prodalumnol Double
21. Rat Death Liquid
22. Chem Pels C
23. Chem-sen 56
24. Caswell No. 744
25. Mfcd00003472
26. Atlas A
27. Arsenite De Sodium
28. Arsenenous Acid, Sodium Salt
29. Arsenite De Sodium [french]
30. Ccris 5554
31. Hsdb 693
32. Einecs 232-070-5
33. Epa Pesticide Chemical Code 013603
34. Unii-48ovy2oc72
35. (naaso2)n
36. Sodium Meta Arsenite
37. Sodium Arsenite [mi]
38. Sodium Arsenite [hsdb]
39. 7784-46-5 (anhydrous)
40. Chembl1909078
41. Dtxsid5020104
42. Sodium (meta)arsenite, >=90%
43. Sodium Arsenite [who-dd]
44. Akos025295751
45. Na(+)n-(-as(o(-))o-)-n
46. Sodium (meta)arsenite, P.a., 98.0%
47. Catena-poly[(oxidoarsenate-mu-oxido)]sodium
48. Sodium Arsenite, 0.5m Standardized Solution
49. Q419586
| Molecular Weight | 129.910 g/mol |
|---|---|
| Molecular Formula | AsNaO2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 129.901193 g/mol |
| Monoisotopic Mass | 129.901193 g/mol |
| Topological Polar Surface Area | 40.1 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 13.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
EXPTL THERAPY THE OPTICAL ISOMERS OF SODIUM 2,3-DIMERCAPTO-1-PROPANESULFONATE WERE SEPARATED AND THE ARSENIC ANTIDOTAL ACTIVITY OF THE L-ISOMER, THE D-ISOMER, AND THE RACEMIC MIXTURE OF 2,3-DIMERCAPTO-1-PROPANESULFONATE WERE INVESTIGATED IN VIVO AND IN VITRO.
PMID:6687396 HAU CA ET AL; J PHARMACOL EXP THER 224 (2): 314-8 (1983)
MEDICATION (VET): TOPICAL ACARICIDE (TECHNICAL GRADE, 90-95% PURE)
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 1356
MEDICATION (VET): COMPONENT OF CATTLE & SHEEP DIPS
SRI
MEDICATION (VET): EXTERNALLY, AS DIP (0.25%) AGAINST LICE & TICKS OF CATTLE, GOATS, & SHEEP. INTERNALLY, IN ORAL ELECTROLYTE MIXT, IN ANTHELMINTICS, & IN ALTERATIVE PREPN WHERE ITS READY WATER SOLUBILITY & RAPID SYSTEMIC ABSORPTION IS DESIRED.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 532
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Arsenite crosses the placenta.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V23 114 (1980)
RATE OF ABSORPTION OF INORG ARSENICALS FROM DIGESTIVE TRACT DEPENDS UPON THEIR SOLUBILITY. SODIUM ARSENITE IS READILY SOL, RAPIDLY ABSORBED.
Clarke, M. L., D. G. Harvey and D. J. Humphreys. Veterinary Toxicology. 2nd ed. London: Bailliere Tindall, 1981., p. 35
AT 20 HR AFTER IV INJECTION OF 4 MG (76)ARSENIC AS SODIUM ARSENITE TO ONE PATIENT WITH TERMINAL CANCER, HIGHEST LEVELS OF ARSENIC WERE FOUND IN LIVER & KIDNEYS & RELATIVELY SMALLER LEVELS IN VARIOUS OTHER TISSUES. EXCRETION OF (76)ARSENIC IN THE FIRST 24 HOURS AFTER AN IV INJECTION OF LABELLED SODIUM ARSENITE TO 2 PATIENTS WITH TERMINAL CANCER WAS 16.7% OF THE INJECTED DOSE; EXCRETION WAS MAINLY VIA THE URINE.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V23 99 (1980)
FOLLOWING IV ADMINISTRATION OF (76)ARSENIC AS SODIUM ARSENITE TO FIVE RATS AND FOUR RABBITS, THE URINARY EXCRETION OF (76)ARSENIC IN THE FIRST 48 HOURS WAS LESS THAN 10% OF THE DOSE IN RATS AND 30% IN RABBITS. FOLLOWING IP INJECTION IN MICE, 75% OF THE DOSE WAS EXCRETED WITHIN THE FIRST 24 HOURS. IN ALL SPECIES TESTED, LESS THAN 10% OF THE TOTAL ... WAS EXCRETED IN THE FECES. UNLIKE RABBITS, RATS RETAIN MOST OF THE INJECTED DOSE IN THE BLOOD FOR A PROLONGED PERIOD. TISSUE DISTRIBUTION STUDIES REVEALED HIGHEST LEVELS OF (76)ARSENIC IN THE BLOOD AND SPLEEN OF RATS, IN THE LIVER, KIDNEYS AND LUNGS OF RABBITS AND IN THE LIVER, KIDNEYS AND SPLEEN OF MICE.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V23 92 (1980)
For more Absorption, Distribution and Excretion (Complete) data for SODIUM ARSENITE (9 total), please visit the HSDB record page.
The urinary metabolites of sodium arsenite have been investigated in rabbits given sodium arsenite and water-soluble dimercaptans. Rabbits injected sc with NaAsO2 (1 mg arsenic/kg) were given im 1 hr later, either saline, 2,3-dimercapto-1-propanesulfonic acid, mesodimercaptosuccinic acid, or N-(2,3-dimercaptopropyl)phthalamidic acid at 0.2 mmol/kg. Arsenic metabolites in urine collected from treated rabbits were isolated by combined anion-cation-exchange chromatography. Column fractions were acid-digested and analyzed for arsenic by direct hydride-flame atomic absorption spectrophotometry. The relative amounts of inorganic arsenic, methylarsonate, and dimethylarsinate found in 0 to 24 hr urine of rabbits given only sodium arsenite agreed closely with those reported for human subjects given arsenite po. This finding suggests that the rabbit biotransforms arsenite in a manner very similar to humans. The urinary excretion of total arsenic between 0 and 24 hr was elevated after dimercaptan administration. but urinary excretion of total arsenic between 0 and 48 hr was unaffected. This result indicates that the action of these dimercaptans occurs early after treatment. In addition, the dimercaptans influenced differently the amounts of the arsenic metabolites excreted in the urine between 0 and 24 hr. 2,3-Dimercapto-1-propanesulfonic acid, mesodimercaptosuccinic acid, or 2,3-dimercapto-1-propanesulfonic acid increased arsenite excretion but decreased dimethylarsinate excretion. 2,3-dimercapto-1-propanesulfonic acid or N-(2,3-dimercaptopropyl)phthalamidic acid treatment increased methylarsonate excretion but mesodimercaptosuccinic acid did not. Arsenate excretion increased after 2,3-dimercapto-1-propanesulfonic acid or mesodimercaptosuccinic acid, treatment but was not affected by N-(2,3-dimercaptopropyl)phthalamidic acid treatment. These results suggest that the dimercaptans, in addition to increasing arsenic excretion, also influence the biotransformation of arsenite to less toxic species. The different effects on the urinary excretion of arsenic metabolites suggest that these dimercaptan metal binding agents have mechanisms of action in addition to simple chelation of inorganic arsenic.
PMID:2983455 Maiorino RM, Aposhian HV; Toxicol Appl Pharmacol 77 (2): 240-250 (1985)
The fungi Candida hemicola biotransforms sodium arsenite to trimethyl arsine.
Cox DP, Alexander M; J Microbiol Ecol 1 (3): 136-144 (1974) as cited in Nat'l Research Council Canada; Effects of Arsenic in the Canadian Environment p.149 (1978) NRCC No. 15391
Cows and dogs were fed sodium arsenite and sodium arsenate daily for five days. Urine was collected and analyzed for methylarsenate and inorganic arsenate. In the cow, the levels rose to 0.1 to 0.5 and 1.0 to 4.0 ppm, respectively. When cows were returned to normal diets, all values returned to control levels (0.02 to 0.10 ppm and 0.1 to 0.2 ppm). In dogs, arsenite feeding produced identical peak values 5.0 to 7.0 ppm for both methylarsenate and inorganic arsenate. Feeding of sodium arsenate to dogs produced a rise to 10 ppm methylarsenate and 5.0 ppm inorganic arsenate. Six days after withdrawal from the arsenic-containing diet, all values reached control levels ... .
Menzie, C.M. Metabolism of Pesticides, Update II. U.S. Department of the Interior, Fish Wildlife Service, Special Scientific Report - Wildlife No. 2l2. Washington, DC: U.S. Government Printing Office, 1978., p. 23
Mice were administered im 1.3 mg As(III)/kg after exposure to a toxic concentration of sodium arsenite (250 mg As(III)/l) for 2, 6 and 8 days. Whereas mice not exposed to the treated drinking water excreted one-half the im administered As(III), those mice previously exposed to the treated water excreted 80% of the applied As(III) as As(V) in 2 days; more than 95%, after 4 days; and after 8 days only traces of As(III) were present ... .
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 34
Cultured BALB/3T3-Cl-A31-1-1 mouse embryo cells were used to study the cytotoxicity, neoplastic transformation, and metabolic reduction or oxidation of trivalent arsenic, in the form of sodium arsenite (arsenic(3+)), and pentavalent arsenic, in the form of sodium arsenate (arsenic(5+)). Uptake of arsenic(3+) and arsenic(5+) by the cells was highest during the first hour and was dose dependent; cells treated with equimolar concn of the agents demonstrated four fold greater uptake for arsenic(3+) than for arsenic(5+). Arsenic(3+) was more cytotoxic than arsenic(5+), although no differences were apparent between the two products with respect to total arsenic in the cells. The relative transformation activity for arsenic(3+) compared to arsenic(5+) was equal to about 4:1. Arsenic(5+) showed a high rate of intracellular metabolic reduction; arsenic(5+) exposure yielded more than 70 percent arsenic(3+) in cytosol, as compared to 100 percent for arsenic(3+) exposure. Ion exchange chromatography did not detect any methylated metabolites. In cell free medium incubations, up to 30 percent of arsenic(3+) was oxidized to arsenic(5+), as compared to only 4 percent oxidation in the presence of cells. Unchanged arsenic(5+) was recovered following its incubation in cell free medium, while the presence of cells resulted in the generation of up to 5 percent arsenic(3+), in a dose dependent manner. The reduction of arsenic(5+) to arsenic(3+) by the cells was inhibited up to 25 percent by depletion of glutathione with diethylmaleate. The authors conclude that arsenic(3+) is responsible for the cytotoxic and transforming action of inorganic arsenic.
PMID:3608077 Bertolero F et al; Carcinogenesis 8 (6): 803-808 (1987)
ARSENITE IS READILY ABSORBED WHEN TAKEN INTERNALLY & POISONING IS ATTRIBUTED TO RAPID DEATH OF CELLS DUE TO INHIBITION OF RESP FROM LACK OF ADENOSINE TRIPHOSPHATE.
PMID:373979 BYARD JL; CLIN TOXICOL 14 (2): 187 (1979)


