
Synopsis
Synopsis
0
CEP/COS
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
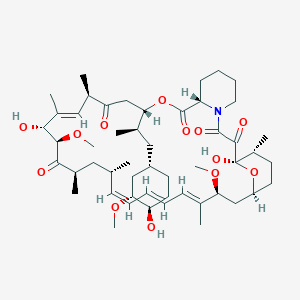

1. Ay 22 989
2. Ay 22-989
3. Ay 22989
4. I 2190a
5. I-2190a
6. I2190a
7. Rapamune
8. Rapamycin
1. (-)-rapamycin
2. 53123-88-9
3. Ay 22989
4. Ay-22989
5. I 2190a
6. I-2190a
7. I2190a
8. Nsc 226080
9. Rapa
10. Rapammune
11. Rapamune
12. Rapamycin
13. Siia 9268a
14. Wy 090217
15. Rapamycin (sirolimus)
16. Rapalimus
17. Wy-090217
18. L04aa10
19. Npc-12g
20. Antibiotic Ay 22989
21. Chebi:9168
22. W36zg6ft64
23. Sm-88 Component Sirolimus
24. De-109
25. Mfcd00867594
26. Nsc-226080
27. Ncgc00021305-05
28. Dsstox_cid_3582
29. Sirolimusum
30. Dsstox_rid_77091
31. Dsstox_gsid_23582
32. (3s,6r,7e,9r,10r,12r,14s,15e,17e,19e,21s,23s,26r,27r,34as)-9,27-dihydroxy-3-{(2r)-1-[(1s,3r,4r)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl}-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-3h-23,27-epoxypyrido[2,1-c][1,4]oxazacyclohentriacontine-1,5,11,28,29(4h,6h,31h)-pentone
33. Sila 9268a
34. Supralimus
35. Rapamycin/sirolimus
36. (1r,9s,12s,15r,16e,18r,19r,21r,23s,24e,26e,28e,30s,32s,35r)-1,18-dihydroxy-12-{(2s)-1-[(1s,3r,4r)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.0(4,9)]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone
37. Cas-53123-88-9
38. Perceiva
39. Cypher
40. Ccris 9024
41. Nsc226080
42. Hsdb 7284
43. 1fkb
44. 1pbk
45. Ncgc00181146-01
46. Lcp-siro
47. (3s,6r,7e,9r,10r,12r,14s,15e,17e,19e,21s,23s,26r,27r,34as)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-9,27-dihydroxy-3-((1r)-2-((1s,3r,4r)-4-hydroxy-3-methoxycyclohexyl)-1-methylethyl)-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3h-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine-1,5,11,28,29(4h,6h,31h)-pentone
48. (3s,6r,7e,9r,10r,12r,14s,15e,17e,19e,21s,23s,26r,27r,34as)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-9,27-dihydroxy-3-[(1r)-2-[(1s,3r,4r)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3h-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(4h,6h,31h)-pentone
49. (3s,6r,7e,9r,10r,12r,14s,15e,17e,19e,21s,23s,26r,27r,34as)-9,27-dihydroxy-3-{(1r)-2-[(1s,3r,4r)-4-hydroxy-3-(methyloxy)cyclohexyl]-1-methylethyl}-6,8,12,14,20,26-hexamethyl-10,21-bis(methyloxy)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-3h-23,27-epoxypyrido[2,1-c][1,4]oxazacyclohentriacontine-1,5,11,28,29(6h,31h)-pentone
50. Rap
51. Fyarro
52. Hyftor
53. S1039
54. Sirolimus [inn]
55. Sirolimus [jan]
56. Rapamycin [mi]
57. Sirolimus [hsdb]
58. Sirolimus [usan]
59. Sirolimus [vandf]
60. Biomolki2_000084
61. Sirolimus [mart.]
62. Rapamycin C-7, Analog 4
63. Schembl3463
64. Sirolimus [usp-rs]
65. Sirolimus [who-dd]
66. Rapamycin,sirolimus,rapamune
67. Unii-w36zg6ft64
68. Bidd:pxr0165
69. Sirolimus [ema Epar]
70. Mls006010168
71. Sirolimus [usan:inn:ban]
72. Gtpl6031
73. Sirolimus [orange Book]
74. Dtxsid5023582
75. Bdbm36609
76. Ms-r001
77. Rapa, Rapamune, Sirolimus, Rpm
78. Hms2089a21
79. Hms3403f11
80. Hms3884c03
81. Ex-a1044
82. Rpm
83. Tox21_110870
84. Tox21_112750
85. Ac-722
86. Bdbm50064359
87. Stl570275
88. Akos015850976
89. Akos015961618
90. Zinc169289388
91. Ccg-100684
92. Cs-0063
93. Db00877
94. Nab-rapamycin Component Rapamycin
95. Nsc-2260804
96. Ncgc00021305-06
97. Ncgc00021305-07
98. Rapamycin From Streptomyces Hygroscopicus
99. As-11687
100. Hy-10219
101. Smr004701276
102. Fyarro (sirolimus Albumin-bound Particles)
103. Everolimus Impurity A [ep Impurity]
104. Unm-0000358684
105. A-275
106. R0097
107. Rapamycin, Vetranal(tm), Analytical Standard
108. Ec 610-965-5
109. M02444
110. Q32089
111. 123r889
112. Q-201659
113. Brd-k84937637-001-04-0
114. Brd-k84937637-001-06-5
115. Brd-k89626439-001-01-0
116. 24,25,26,27,32,33,34,34a-hexadecahydro-3h-23,27-epoxypy
117. Rapamycin From Streptomyces Hygroscopicus, >=95% (hplc), Powder
118. Rapamycin From Streptomyces Hygroscopicus, Vetec(tm) Reagent Grade, >=95%
119. Sirolimus Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
120. (1r,9s,12s,15r,16e,18r,19r,21r,23s,24e,26e,28e,30s,32s,35r)-1,18-dihydroxy-12-{(2s)-1-[(1s,3r,4r)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone
121. (3s,6r,7e,9r,10r,12r,14s,15e,17e,19e,21s,23s,26r,27r,34 As)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hex Adecahydro-9,27-dihydroxy-3-[(1r)-2-[(1s,3r,4r)-4-hydro Xy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy- 6,8,12,14,20,26-hexamethyl-23,27-ep
122. (3s,6r,7e,9r,10r,12r,14s,15e,17e,19e,21s,23s,26r,27r,34as)-4,9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-heptadecahydro-9,27-dihydroxy-3-[(1r)-2-[(1s,3,4r)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-223,27-epoxy-3h-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(6h,31h)-pentone
123. (3s,6r,7e,9r,10r,12r,14s,15e,17e,19e,21s,23s,26r,27r,34as)-9,27-dihydroxy-3-{(1r)-2-[(1s,3r,4r)-4-hydroxy-3-(methyloxy)cyclohexyl]-1-methylethyl}-6,8,12,14,20,26-hexamethyl-10,21-bis(methyloxy)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro
124. (3s,6r,7e,9r,10r,12r,14s,15e,17e,19e,21s,23s,26r,27r,34as)-9,27-dihydroxy-3-{(1r)-2-[(1s,3r,4r)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl}-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-3h-23,27-epoxypyrido[2,1-c][1,4]oxazacyclohentriacontine-1,5,11,28,29(4h,6h,31h)-pentone
125. (3s,6r,7e,9r,10r,12r,14s,15e,17e,19e,21s,23s,26r,27r,34as)-9,27-dihydroxy-3-{1-[(1s,3r,4r)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl}-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-9,10,12,13,14,21,22,23,
126. 1,18-dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone (rapamycin)
1. (rs)-rapamycin
| Molecular Weight | 914.2 g/mol |
|---|---|
| Molecular Formula | C51H79NO13 |
| XLogP3 | 6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 6 |
| Exact Mass | 913.55514157 g/mol |
| Monoisotopic Mass | 913.55514157 g/mol |
| Topological Polar Surface Area | 195 Ų |
| Heavy Atom Count | 65 |
| Formal Charge | 0 |
| Complexity | 1760 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 15 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Rapamune |
| PubMed Health | Sirolimus (By mouth) |
| Drug Classes | Immune Suppressant |
| Drug Label | Rapamune (sirolimus) is an immunosuppressive agent. Sirolimus is a macrocyclic lactone produced by Streptomyces hygroscopicus. The chemical name of sirolimus (also known as rapamycin) is (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10... |
| Active Ingredient | Sirolimus |
| Dosage Form | Tablet; Solution |
| Route | Oral |
| Strength | 1mg/ml; 0.5mg; 1mg; 2mg |
| Market Status | Prescription |
| Company | Pf Prism Cv |
| 2 of 4 | |
|---|---|
| Drug Name | Sirolimus |
| PubMed Health | Sirolimus (By mouth) |
| Drug Classes | Immune Suppressant |
| Drug Label | Rapamune (sirolimus) is an immunosuppressive agent. Sirolimus is a macrocyclic lactone produced by Streptomyces hygroscopicus. The chemical name of sirolimus (also known as rapamycin) is (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10... |
| Active Ingredient | Sirolimus |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 0.5mg; 1mg; 2mg |
| Market Status | Tentative Approval; Prescription |
| Company | Zydus Pharms Usa; Dr Reddys Labs |
| 3 of 4 | |
|---|---|
| Drug Name | Rapamune |
| PubMed Health | Sirolimus (By mouth) |
| Drug Classes | Immune Suppressant |
| Drug Label | Rapamune (sirolimus) is an immunosuppressive agent. Sirolimus is a macrocyclic lactone produced by Streptomyces hygroscopicus. The chemical name of sirolimus (also known as rapamycin) is (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10... |
| Active Ingredient | Sirolimus |
| Dosage Form | Tablet; Solution |
| Route | Oral |
| Strength | 1mg/ml; 0.5mg; 1mg; 2mg |
| Market Status | Prescription |
| Company | Pf Prism Cv |
| 4 of 4 | |
|---|---|
| Drug Name | Sirolimus |
| PubMed Health | Sirolimus (By mouth) |
| Drug Classes | Immune Suppressant |
| Drug Label | Rapamune (sirolimus) is an immunosuppressive agent. Sirolimus is a macrocyclic lactone produced by Streptomyces hygroscopicus. The chemical name of sirolimus (also known as rapamycin) is (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10... |
| Active Ingredient | Sirolimus |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 0.5mg; 1mg; 2mg |
| Market Status | Tentative Approval; Prescription |
| Company | Zydus Pharms Usa; Dr Reddys Labs |
Sirolimus is indicated for the prevention of rejection of transplanted kidney allografts. It is recommended that sirolimus be used in a regimen with cyclosporine and corticosteroids. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2546
Long-term results after percutaneous coronary intervention in the treatment of chronic total coronary occlusions is hindered by a significant rate of restenosis and reocclusion. In the treatment of relatively simple nonocclusive lesions, sirolimus-eluting stents have shown dramatically reduced restenosis rates compared with bare metal stents, but whether these results are more widely applicable is unknown. ... The use of sirolimus-eluting stents in the treatment of chronic total coronary occlusions is associated with a reduction in the rate of major adverse cardiac events and restenosis compared with bare metal stents.
PMID:15172397 Hoye A et al; J Am Coll Cardiol 43 (11): 1954-8 (2004)
Chronic renal failure triggered by calcineurin inhibitor (CNI)-based immunosuppression is a common complication after cardiac transplantation. Sirolimus and mycophenolate mofetil (MMF) are 2 newer immunosuppressive agents with no documented nephrotoxic side effects. This case report describes a patient with ongoing chronic renal failure 10 months after cardiac transplantation on cyclosporine-based immunosuppressive therapy. Conversion of the immunosuppressive regimen from cyclosporine to sirolimus and MMF resulted in freedom from acute rejection, excellent cardiac graft function and consistently improved renal function. This case illustrates the beneficial potential of sirolimus and MMF as CNI-free and safe long-term immunosuppression in a patient with chronic renal failure after heart transplantation.
PMID:15366440 Groetzner J et al; J Heart Lung Transplant 23 (6): 770-3 (2004)
/BOXED WARNING/ IMMUNOSUPPRESSION, USE IS NOT RECOMMENDED IN LIVER OR LUNG TRANSPLANT PATIENTS. Increased susceptibility to infection and the possible development of lymphoma and other malignancies may result from immunosuppression Increased susceptibility to infection and the possible development of lymphoma may result from immunosuppression. Only physicians experienced in immunosuppressive therapy and management of renal transplant patients should use Rapamune. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient. The safety and efficacy of Rapamune (sirolimus) as immunosuppressive therapy have not been established in liver or lung transplant patients, and therefore, such use is not recommended. Liver Transplantation - Excess Mortality, Graft Loss, and Hepatic Artery Thrombosis (HAT): The use of Rapamune in combination with tacrolimus was associated with excess mortality and graft loss in a study in de novo liver transplant patients. Many of these patients had evidence of infection at or near the time of death. In this and another study in de novo liver transplant patients, the use of Rapamune in combination with cyclosporine or tacrolimus was associated with an increase in HAT; most cases of HAT occurred within 30 days post-transplantation and most led to graft loss or death. Lung Transplantation - Bronchial Anastomotic Dehiscence: Cases of bronchial anastomotic dehiscence, most fatal, have been reported in de novo lung transplant patients when Rapamune has been used as part of an immunosuppressive regimen.
US Natl Inst Health; DailyMed. Current Medication Information for Rapamune (sirolimus) solution; Rapamune (sirolimus) tablet, sugar coated (Updated: March 2015). Available from, as of April 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3275b824-3f82-4151-2ab2-0036a9ba0acc
Grapefruit juice may inhibit CYP 3A4 enzymes, leading to decreased metabolism of sirolimus; must not be taken with or used to dilute sirolimus.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2547
Cases of bronchial anastomotic dehiscence, most of which were fatal, have been reported in de novo lung transplant patients who received sirolimus in combination with other immunosuppressants. Because safety and efficacy of sirolimus as immunosuppressive therapy in lung transplant patients have not been established, such use in not recommended by the manufacturer.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 3645
Use of sirolimus in combination with other immunosuppressants (i.e., cyclosporine, tacrolimus) has been associated with an increased risk on hepatic artery thrombosis, graft loss, and death in de novo liver transplant recipients. Because safety and efficacy of sirolimus as immunosuppressive therapy in liver transplant patients have not been established, such use is not recommended by the manufacturer.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 3646
For more Drug Warnings (Complete) data for SIROLIMUS (27 total), please visit the HSDB record page.
Sirolimus is indicated for the prophylaxis of organ rejection in patients aged 13 years or older receiving renal transplants. In patients at low-to moderate-immunologic risk, it is recommended that sirolimus be used initially in a regimen with [cyclosporine] and corticosteroids; cyclosporine should be withdrawn two to four months after transplantation. In patients at high-immunologic risk (defined as Black recipients and/or repeat renal transplant recipients who lost a previous allograft for immunologic reason and/or patients with high panel-reactive antibodies [PRA; peak PRA level > 80%]), it is recommended that sirolimus be used in combination with cyclosporine and corticosteroids for the first year following transplantation. It is also used to treat lymphangioleiomyomatosis. In the US, albumin-bound sirolimus for intravenous injection is indicated for the treatment of adult patients with locally advanced unresectable or metastatic malignant perivascular epithelioid cell tumour (PEComa). In Europe, it is recommended that sirolimus for the prophylaxis of organ rejection in renal transplants is used in combination with cyclosporin microemulsion and corticosteroids for two to three months. Sirolimus may be continued as maintenance therapy with corticosteroids only if cyclosporin microemulsion can be progressively discontinued.
FDA Label
Rapamune is indicated for the prophylaxis of organ rejection in adult patients at low to moderate immunological risk receiving a renal transplant. It is recommended that Rapamune be used initially in combination with ciclosporin microemulsion and corticosteroids for 2 to 3 months. Rapamune may be continued as maintenance therapy with corticosteroids only if ciclosporin microemulsion can be progressively discontinued.
Rapamune is indicated for the treatment of patients with sporadic lymphangioleiomyomatosis with moderate lung disease or declining lung function.
Prevention of arteriovenous access dysfunction
Treatment of chronic non-infectious uveitis
Sirolimus is an immunosuppressant drug with antifungal and antitumour effects. In animal models, sirolimus prolonged allograft survival following various organ transplants and reversed an acute rejection of heart and kidney allografts in rats. Upon oral administration of 2 mg/day and 5 mg/day, sirolimus significantly reduced the incidence of organ rejection in low- to moderate-immunologic risk renal transplant patients at six months following transplantation compared with either azathioprine or placebo. In some studies, the immunosuppressive effect of sirolimus lasted up to six months after discontinuation of therapy: this tolerization effect is alloantigen-specific. Sirolimus potently inhibits antigen-induced proliferation of T cells, B cells, and antibody production. In rodent models of autoimmune disease, sirolimus suppressed immune-mediated events associated with systemic lupus erythematosus, collagen-induced arthritis, autoimmune type I diabetes, autoimmune myocarditis, experimental allergic encephalomyelitis, graft-versus-host disease, and autoimmune uveoretinitis.
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
L04AA10
L04AA10
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA10 - Sirolimus
S - Sensory organs
S01 - Ophthalmologicals
S01X - Other ophthalmologicals
S01XA - Other ophthalmologicals
S01XA23 - Sirolimus
Absorption
In adult renal transplant patients with low- to moderate-immunologic risk, oral administration of 2 mg sirolimus led to a Cmax of 14.4 5.3 ng/mL for oral solution and 15.0 4.9 ng/mL for oral tablets. The tmax was 2.1 0.8 hours for oral solution and 3.5 2.4 hours for oral tablets. In healthy subjects, the tmax is one hour. In a multi-dose study, steady-state was reached six days following repeated twice-daily administration without an initial loading dose, with the average trough concentration of sirolimus increased approximately 2- to 3-fold. It is suspected that a loading dose of three times the maintenance dose will provide near steady-state concentrations within one day in most patients. The systemic availability of sirolimus is approximately 14%. In healthy subjects, the mean bioavailability of sirolimus after administration of the tablet is approximately 27% higher relative to the solution. Sirolimus tablets are not bioequivalent to the solution; however, clinical equivalence has been demonstrated at the 2 mg dose level. Sirolimus concentrations, following the administration of Rapamune Oral Solution to stable renal transplant patients, are dose-proportional between 3 and 12 mg/m2.
Route of Elimination
Following oral administration of [14C] sirolimus in healthy subjects, about 91% of the radioactivity was recovered from feces and only 2.2% of the radioactivity was detected in urine. Some of the metabolites of sirolimus are also detectable in feces and urine.
Volume of Distribution
The mean ( SD) blood-to-plasma ratio of sirolimus was 36 18 L in stable renal allograft patients, indicating that sirolimus is extensively partitioned into formed blood elements. The mean volume of distribution (Vss/F) of sirolimus is 12 8 L/kg.
Clearance
In adult renal transplant patients with low- to moderate-immunologic risk, oral administration of 2 mg sirolimus led to oral clearance of 173 50 mL/h/kg for oral solution and 139 63 mL/h/kg for oral tablets.
Following administration of /Sirolimus/ Oral Solution, sirolimus is rapidly absorbed, with a mean time-to-peak concentration (t max ) of approximately 1 hour after a single dose in healthy subjects and approximately 2 hours after multiple oral doses in renal transplant recipients. The systemic availability of sirolimus was estimated to be approximately 14% after the administration of /Sirolimus/ Oral Solution. The mean bioavailability of sirolimus after administration of the tablet is about 27% higher relative to the oral solution.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 3483
In 22 healthy volunteers receiving Rapamune Oral Solution, a high-fat meal altered the bioavailability characteristics of sirolimus. Compared with fasting, a 34% decrease in the peak blood sirolimus concentration (C max ), a 3.5-fold increase in the time-to-peak concentration (t max ), and a 35% increase in total exposure (AUC) was observed. After administration of Rapamune Tablets and a high-fat meal in 24 healthy volunteers, C max , t max , and AUC showed increases of 65%, 32%, and 23%, respectively.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 3483
Absorption: Rapid, from the gastrointestinal tract. Bioavailability is approximately 14%. Rate of absorption is decreased in the presence of a high-fat diet. The rate and extent of absorption is reduced in black patients.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2546
The mean (+/- SD) blood-to-plasma ratio of sirolimus was 36 +/- 17.9 in stable renal allograft recipients, indicating that sirolimus is extensively partitioned into formed blood elements. The mean volume of distribution of sirolimus is 12 +/- 7.52 L/kg. Sirolimus is extensively bound (approximately 92%) to human plasma proteins. In man, the binding of sirolimus was shown mainly to be associated with serum albumin (97%), (alpha) 1 -acid glycoprotein, and lipoproteins.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 3483
For more Absorption, Distribution and Excretion (Complete) data for SIROLIMUS (7 total), please visit the HSDB record page.
Sirolimus undergoes extensive metabolism in the intestinal wall and liver. Sirolimus is primarily metabolized by O-demethylation and/or hydroxylation via CYP3A4 to form seven major metabolites, including hydroxy, demethyl, and hydroxydemethyl metabolites, which are pharmacologically inactive. Sirolimus also undergoes counter-transport from enterocytes of the small intestine into the gut lumen.
Sirolimus is a substrate for both cytochrome P450 IIIA4 (CYP3A4) and P-glycoprotein. Sirolimus is extensively metabolized by O-demethylation and/or hydroxylation. Seven major metabolites, including hydroxy, demethyl, and hydroxydemethyl, are identifiable in whole blood. Some of these metabolites are also detectable in plasma, fecal, and urine samples. Glucuronide and sulfate conjugates are not present in any of the biologic matrices.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 3484
Biotransformation: Hepatic, extensive, by cytochrome p450 3A enzymes. Major metabolites include hydroxysirolimus, demethylsirolimus, and hydroxydemethyl-sirolimus.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2546
... After incubation of sirolimus with human and pig small intestinal microsomes, five metabolites were detected using high performance liquid chromatography/electrospray-mass spectrometry: hydroxy, dihydroxy, trihydroxy, desmethyl and didesmethyl sirolimus. The same metabolites were generated by human liver microsomes and pig small intestinal mucosa in the Ussing chamber. Anti-CYP3A antibodies, as well as the specific CYP3A inhibitors troleandomycin and erythromycin, inhibited small intestinal metabolism of sirolimus, confirming that, as in the liver, CYP3A enzymes are responsible for sirolimus metabolism in the small intestine. ...
PMID:9618413 Lampen A et al; J Pharmacol Exp Ther 285 (3): 1104-12 (1998)
Sirolimus has known human metabolites that include 11-Hydroxy-sirolimus, 12-Hydroxy-sirolimus, 16-O-Desmethylsirolimus, 24-Hydroxy-sirolimus, 25-Hydroxy-sirolimus, 39-O-Desmethylsirolimus, and 46-Hydroxy-sirolimus.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The mean SD terminal elimination half-life (t) of sirolimus after multiple dosing in stable renal transplant patients was estimated to be about 62 16 hours.
The drug has an elimination half life of 57-63 hours in kidney transplant recipients.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 3647
Sirolimus works by inhibiting T-lymphocyte activation and proliferation stimulated by antigens and cytokines such as interleukin (IL)-2, IL-4, and IL-15. In target cells, sirolimus binds to the cytoplasmic receptor FK506-binding protein-12 (FKBP12), an immunophilin, to form an immunosuppressive complex. FKBP12-sirolimus complex binds to and inhibits the activation of the mammalian target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that regulates cell growth, proliferation, survival, mobility, and angiogenesis. mTOR regulates the downstream signalling pathways involved in cell survival, such as the phosphatidylinositol-3 kinase (PI3K)/Akt signalling pathway. Inhibition of mTOR leads to the suppression of cytokine-driven T-cell proliferation, thus the progression from the G1 to the S phase of the cell cycle is inhibited. Sirolimus also inhibits antibody production. _In vitro_, sirolimus and other mTOR inhibitors inhibit the production of certain growth factors that may affect angiogenesis, fibroblast proliferation, and vascular permeability. Lymphangioleiomyomatosis is a disorder that primarily affects the lungs. It is characterized by lung tissue infiltration, unregulated alveolar smooth muscle proliferation, and cystic destruction of parenchyma. Although infrequent, it occurs as a symptomatic pulmonary complication in tuberous sclerosis complex (TSC), which is an inherited disorder caused by mutations in TSC genes. Loss of functional TSC gene leads to the aberrant activation of the mTOR signalling pathway, resulting in cellular proliferation and release of lymphangiogenic growth factors. Sirolimus inhibits the activated mTOR pathway and proliferation of alveolar smooth muscle cell proliferation.
Sirolimus inhibits cytokine (Interleukin (IL)-2, IL-4, and IL-15) -- stimulated T lymphocyte activation and proliferation; it also inhibits antibody production. This may occur through formation of an immunosuppressive complex with FK Binding Protein-12 (FKBP-12). Although the sirolimus-(FKBP-12) complex is inactive against calcineurin activity, the complex binds to and inhibits activation of a key regulatory kinase, mammalian Target of Rapamycin (mTOR). This is believed to suppress cytokine-driven T-cell proliferation, inhibiting cell cycle progression from the G, to S phase.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2546

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
37
PharmaCompass offers a list of Sirolimus API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sirolimus manufacturer or Sirolimus supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sirolimus manufacturer or Sirolimus supplier.
PharmaCompass also assists you with knowing the Sirolimus API Price utilized in the formulation of products. Sirolimus API Price is not always fixed or binding as the Sirolimus Price is obtained through a variety of data sources. The Sirolimus Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sirolimus manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sirolimus, including repackagers and relabelers. The FDA regulates Sirolimus manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sirolimus API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Sirolimus manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Sirolimus supplier is an individual or a company that provides Sirolimus active pharmaceutical ingredient (API) or Sirolimus finished formulations upon request. The Sirolimus suppliers may include Sirolimus API manufacturers, exporters, distributors and traders.
click here to find a list of Sirolimus suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sirolimus DMF (Drug Master File) is a document detailing the whole manufacturing process of Sirolimus active pharmaceutical ingredient (API) in detail. Different forms of Sirolimus DMFs exist exist since differing nations have different regulations, such as Sirolimus USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Sirolimus DMF submitted to regulatory agencies in the US is known as a USDMF. Sirolimus USDMF includes data on Sirolimus's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Sirolimus USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Sirolimus suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Sirolimus Drug Master File in Japan (Sirolimus JDMF) empowers Sirolimus API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Sirolimus JDMF during the approval evaluation for pharmaceutical products. At the time of Sirolimus JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Sirolimus suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Sirolimus Drug Master File in Korea (Sirolimus KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Sirolimus. The MFDS reviews the Sirolimus KDMF as part of the drug registration process and uses the information provided in the Sirolimus KDMF to evaluate the safety and efficacy of the drug.
After submitting a Sirolimus KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Sirolimus API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Sirolimus suppliers with KDMF on PharmaCompass.
A Sirolimus written confirmation (Sirolimus WC) is an official document issued by a regulatory agency to a Sirolimus manufacturer, verifying that the manufacturing facility of a Sirolimus active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Sirolimus APIs or Sirolimus finished pharmaceutical products to another nation, regulatory agencies frequently require a Sirolimus WC (written confirmation) as part of the regulatory process.
click here to find a list of Sirolimus suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Sirolimus as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Sirolimus API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Sirolimus as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Sirolimus and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Sirolimus NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Sirolimus suppliers with NDC on PharmaCompass.
Sirolimus Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sirolimus GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sirolimus GMP manufacturer or Sirolimus GMP API supplier for your needs.
A Sirolimus CoA (Certificate of Analysis) is a formal document that attests to Sirolimus's compliance with Sirolimus specifications and serves as a tool for batch-level quality control.
Sirolimus CoA mostly includes findings from lab analyses of a specific batch. For each Sirolimus CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sirolimus may be tested according to a variety of international standards, such as European Pharmacopoeia (Sirolimus EP), Sirolimus JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sirolimus USP).

