
Synopsis
Synopsis
0
VMF
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
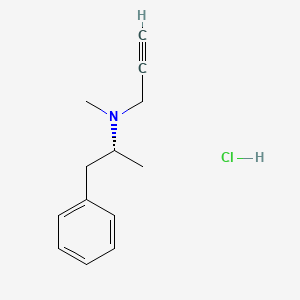

1. Deprenalin
2. Deprenil
3. Deprenyl
4. E 250
5. E-250
6. E250
7. Eldepryl
8. Emsam
9. Humex
10. Jumex
11. L-deprenyl
12. Selegiline
13. Selegiline Hydrochloride, (r)-isomer
14. Selegiline Hydrochloride, (r,s)-isomer
15. Selegiline Hydrochloride, (s)-isomer
16. Selegiline, (r)-isomer
17. Selegiline, (r,s)-isomer
18. Selegiline, (s)-isomer
19. Selegyline
20. Yumex
21. Zelapar
1. 14611-52-0
2. Eldepryl
3. Selegiline Hcl
4. Zelapar
5. L-deprenyl Hydrochloride
6. (-)-deprenyl Hydrochloride
7. L-deprenyl
8. Ensam
9. (r)-n-methyl-n-(1-phenylpropan-2-yl)prop-2-yn-1-amine Hydrochloride
10. R-(-)-deprenyl Hydrochloride
11. Jumex
12. R-(-)-deprenyl (hydrochloride)
13. R(-)-deprenyl Hydrochloride
14. (r)-(-)-deprenyl Hydrochloride
15. (-)-phenylisopropylmethylpropynylamine
16. Otrasel
17. 6w731x367q
18. Nsc-759259
19. Benzeneethanamine, N,alpha-dimethyl-n-2-propyn-1-yl-, Hydrochloride (1:1), (alphar)-
20. Dsstox_cid_24584
21. Dsstox_rid_80331
22. Jumex Hydrochloride
23. Dsstox_gsid_44584
24. Eldepryl Hydrochloride
25. Zydis Selegiline
26. (2r)-n-methyl-1-phenyl-n-prop-2-ynylpropan-2-amine;hydrochloride
27. Chebi:9087
28. (-)-deprenil Hydrochloride
29. (-)-e-250 Hydrochloride
30. Smr000449328
31. Selegiline Hydrochloride [usan]
32. Hsdb 7183
33. Fpf1100
34. Sr-01000597928
35. Plurimen
36. Seledat
37. Vivapryl
38. Xilopar
39. Ccris 8571
40. Unii-6w731x367q
41. Eldepryl (tn)
42. Prestwick_846
43. (r)-deprenyl Hcl
44. Selegiline Hydrochloride [usan:usp]
45. (-)-(r)-n,alpha-dimethyl-n-2-propynylphenethylamine Hydrochloride
46. (-)-n,alpha-dimethyl-n-2-propynylbenzeneethanamine Hydrochloride
47. Ncgc00016708-01
48. (-)-(r)-n,alpha-dimethyl-n-2-propynylphenethylamine Monohydrochloride
49. Cas-14611-52-0
50. R-(-)deprenyl Hydrochloride
51. Schembl41392
52. (-)-selegiline Hydrochloride
53. Mls000758294
54. Mls001423947
55. Mls002153281
56. Mls002222269
57. Chembl1200904
58. Dtxsid9044584
59. Hms1569p05
60. Fpf-1100
61. Selegiline Hydrochloride (jan/usp)
62. Tox21_110572
63. Tox21_301846
64. (r)-(-)-n,alpha-dimethyl-n-(2-propynyl)phenethylamine Hydrochloride
65. Mfcd00069299
66. Selegiline Hydrochloride [mi]
67. Selegiline Hydrochloride [jan]
68. Akos005166822
69. Benzeneethanamine, N,.alpha.-dimethyl-n-2-propynyl-, Hydrochloride, (r)-
70. Benzeneethanamine, N,alpha-dimethyl-n-2-propynyl-, Hydrochloride, (r)-
71. Tox21_110572_1
72. Ccg-100773
73. Ks-5098
74. Nc00023
75. Nc00605
76. Nsc 759259
77. Selegiline Hydrochloride [hsdb]
78. Selegiline Hydrochloride [mart.]
79. Selegiline Hydrochloride [vandf]
80. Ncgc00024994-06
81. Ncgc00255797-01
82. Selegiline Hydrochloride [usp-rs]
83. Selegiline Hydrochloride [who-dd]
84. Ac-18759
85. Hy-14199
86. Selegiline Hydrochloride [green Book]
87. Selegiline Hydrochloride [orange Book]
88. D00785
89. Selegiline Hydrochloride [ep Monograph]
90. Selegiline Hydrochloride [usp Monograph]
91. 611s520
92. Sr-01000597928-1
93. Sr-01000597928-5
94. Sr-01000597928-6
95. Q27108267
96. R-(-)-deprenyl Hydrochloride, Powder, >=98% (hplc)
97. Selegiline Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
98. (r)-(-)-n,alpha-dimethyl-n-(2-propynyl)phenethylamine Hcl
99. (r)-(-)-n-?-dimethyl-n-2-propynylbenzeneethanamine Hydrochloride
100. (r)-n-methyl-1-phenyl-n-prop-2-ynylpropan-2-amine Hydrochloride
101. (-)-(r)-n,.alpha.-dimethyl-n-2-propynylphenethylamine Hydrochloride
102. Selegiline Hydrochloride, European Pharmacopoeia (ep) Reference Standard
103. Benzeneethanamine, N,?-dimethyl-n-2-propyn-1-yl-, Hydrochloride (1:1), (?r)-
104. Selegiline Hydrochloride, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 223.74 g/mol |
|---|---|
| Molecular Formula | C13H18ClN |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 4 |
| Exact Mass | 223.1127773 g/mol |
| Monoisotopic Mass | 223.1127773 g/mol |
| Topological Polar Surface Area | 3.2 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 195 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Eldepryl |
| Drug Label | ELDEPRYL (selegiline hydrochloride) is a levorotatory acetylenic derivative of phenethylamine. It is commonly referred to in the clinical and pharmacological literature as l-deprenyl.The chemical name is: (R)-(-)-N,2-dimethyl-N-2-propynylphenethylami... |
| Active Ingredient | Selegiline hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 5mg |
| Market Status | Prescription |
| Company | Somerset |
| 2 of 6 | |
|---|---|
| Drug Name | Selegiline hydrochloride |
| PubMed Health | Selegiline (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | Selegiline hydrochloride is a levorotatory acetylenic derivative of phenethylamine. It is commonly referred to in the clinical and pharmacological literature as l-deprenyl. The chemical name is: (R)-(-)-N,2-dimethyl-N-2-propynylphenethylamine hydroch... |
| Active Ingredient | Selegiline hydrochloride |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 5mg |
| Market Status | Prescription |
| Company | Apotex; Stason; Dava Pharms; Mylan |
| 3 of 6 | |
|---|---|
| Drug Name | Zelapar |
| PubMed Health | Selegiline |
| Drug Classes | Antidepressant, Antiparkinsonian |
| Drug Label | ZELAPAR Orally Disintegrating Tablets contain selegiline hydrochloride, a levorotatory acetylenic derivative of phenthylamine. Selegiline hydrochloride is described chemically as: (-)-(R)-N, -dimethyl-N-2-propynylphenethylamine hydrochloride and it... |
| Active Ingredient | Selegiline hydrochloride |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 1.25mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 4 of 6 | |
|---|---|
| Drug Name | Eldepryl |
| Drug Label | ELDEPRYL (selegiline hydrochloride) is a levorotatory acetylenic derivative of phenethylamine. It is commonly referred to in the clinical and pharmacological literature as l-deprenyl.The chemical name is: (R)-(-)-N,2-dimethyl-N-2-propynylphenethylami... |
| Active Ingredient | Selegiline hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 5mg |
| Market Status | Prescription |
| Company | Somerset |
| 5 of 6 | |
|---|---|
| Drug Name | Selegiline hydrochloride |
| PubMed Health | Selegiline (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | Selegiline hydrochloride is a levorotatory acetylenic derivative of phenethylamine. It is commonly referred to in the clinical and pharmacological literature as l-deprenyl. The chemical name is: (R)-(-)-N,2-dimethyl-N-2-propynylphenethylamine hydroch... |
| Active Ingredient | Selegiline hydrochloride |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 5mg |
| Market Status | Prescription |
| Company | Apotex; Stason; Dava Pharms; Mylan |
| 6 of 6 | |
|---|---|
| Drug Name | Zelapar |
| PubMed Health | Selegiline |
| Drug Classes | Antidepressant, Antiparkinsonian |
| Drug Label | ZELAPAR Orally Disintegrating Tablets contain selegiline hydrochloride, a levorotatory acetylenic derivative of phenthylamine. Selegiline hydrochloride is described chemically as: (-)-(R)-N, -dimethyl-N-2-propynylphenethylamine hydrochloride and it... |
| Active Ingredient | Selegiline hydrochloride |
| Dosage Form | Tablet, orally disintegrating |
| Route | Oral |
| Strength | 1.25mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
Monamine oxidase B inhibitor
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1512
Selegiline is indicated for use with levodopa or levodopa and carbidopa combination in the treatment of idiopathic Parkinson's disease (paralysis agitans). /Included in US product labeling/ /Selegiline/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2442
.... we report briefly on neurotoxicity associated with the immunodeficiency virus and discuss the effects of selegiline, a monoamine oxidase inhibitor which enhances dopamine availability in CNS on immunodeficiency virus-induced neurological disease. ... /MAO inhibitors may be potent mediators of the neuropathological deficits in immunodeficiency virus infection and factors which may accelerate the progression of the immunodeficiency virus --neurological disease./
PMID:14697901 Koutsilieri E et al; Neurotoxicology 25 (1-2): 267-70 (2004)
EXPTL Therapy: ... Twenty patients with mild-moderate pathological cerebral involution of atrophic and/or vascular origin, were treated with Selegiline (L-deprenyl), a monoamino-oxidase B inhibitor (10 mg/day for six months). Compared with a control group, Selegiline treated patients showed a statistically significant improvement in cognitive and behaviour capacities. At the end of investigation, "Mini Mental State" showed an improvement of 26.5% in Selegiline group and of 3.7% in control group (P < 0.01). "Echelle Clinique d'Aptitudes Intellectuelles" showed an improvement of 29.4% and of 10.8% respectively (P < 0.01). Selegiline treatment has shortened significantly the reaction times and has improved mnesic capacities. No side effects were observed during the study. /Selegiline/
PMID:12645393 Bettini R, Gorini M; Clin Ter 153 (6): 377-80 (2002)
For more Therapeutic Uses (Complete) data for SELEGILINE HYDROCHLORIDE (10 total), please visit the HSDB record page.
Pregnancy risk category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 3176
Selegiline may decrease or inhibit salivary flow, thus contributing to the development of caries, periodontal disease, oral candidiasis, and discomfort.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2443
Therapeutic use may lead to increased tremor, bradykinesia, falling, dystonic symptoms, dyskinesias, hallucinations, confusion, sleep disturbance, headache, dry mouth, blurred vision, orthostatic hypotension, hypertension, poor appetite, urinary retention, and diaphoresis. Hypomanic behavior may be due to the l-amphetamine and l-amphetamine metabolites of selegiline.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 659
In patients receiving levodopa, addition of selegiline hydrochloride may exacerbate levodopa-associated dyskinesias. This effect, which occurred in an average of 28% (range: 4-90%) of patients receiving the drug in clinical trials, usually occurs within 2 weeks after initiating selegiline therapy and generally is mitigated when the levodopa dosage is reduced ... . Involuntary movements, increased tremor, chorea, loss of balance, freezing, blepharospasm, increased bradykinesia, facial grimacing, speech problems, heavy leg, stiff neck, tardive dyskinesia, dystonic manifestations, festination, increased apraxia, and muscle cramping may occur in patients receiving selegiline. Bruxism, muscle twitching and myoclonic jerks have occurred in patients receiving levodopa and selegiline hydrochloride dosages exceeding 10 mg daily.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2439
For more Drug Warnings (Complete) data for SELEGILINE HYDROCHLORIDE (12 total), please visit the HSDB record page.
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
Monoamine Oxidase Inhibitors
A chemically heterogeneous group of drugs that have in common the ability to block oxidative deamination of naturally occurring monoamines. (From Gilman, et al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed, p414) (See all compounds classified as Monoamine Oxidase Inhibitors.)
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
Antiparkinson Agents
Agents used in the treatment of Parkinson's disease. The most commonly used drugs act on the dopaminergic system in the striatum and basal ganglia or are centrally acting muscarinic antagonists. (See all compounds classified as Antiparkinson Agents.)
Selegiline and its metabolites are widely distributed into body tissues and cross the blood-brain barrier. Following IV administration of radiolabeled selegiline hydrochloride in mice, the parent drug and/or metabolites are rapidly and widely distributed to brain, liver, kidney, lung, heart, and brown fat. Following IV administration of radiolabeled selegiline hydrochloride in healthy adults, the highest accumulation of radioactivity occurred in the thalamus, basal ganglia, mesencephalon, and cingulate gyrus. /Selegiline/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2441
Selegiline is excreted principally in urine as conjugated and unconjugated metabolites. About 20-63% of an orally administered dose of selegiline is excreted in urine as l-methamphetamine, 9-26% as l-amphetamine, and 1% as l-demethylselegiline. ... About 15% of a dose is excreted in feces within 72 hours following administration of selegiline. /SRP: This would usually result in a false positive drug test for d-methamphetamine if no d/l-isomer characterization was performed on the specimen./ /Selegiline/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2441
Rapidly absorbed from the gastrointestinal tract.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2442
... The mean peak plasma concentration (Cmax) is approximately 2 ug/L and the time to reach the peak is under an hour. The absolute bioavailability of selegiline is approximately 10%. It has an apparent volume of distribution of 1854 L. The oral clearance of selegiline (59 L/min) is many fold higher than the liver blood flow (1.5 L/min), indicating that extrahepatic processes are involved in the elimination of selegiline. ... /Selegiline/
PMID:9260033 Mahmood I; Clin Pharmacokinet 33 (2): 91-102 (1997)
... Selegiline is readily absorbed from the gastrointestinal tract and rapidly enters the brain and spinal cord following oral administration. The drug binds to brain regions with a high MAO-B content, such as the thalamus, the striatum, the cortex, and the brainstem. ... /Selegiline/
Gerlach M et al; Neurology 47 (6 Suppl 3): S137-45 (1996)
Selegiline is excreted principally in urine as conjugated and unconjugated metabolites. About 20-63% of an orally administered dose of selegiline is excreted in urine as l-methamphetamine, 9-26% as l-amphetamine, and 1% as l-demethylselegiline. /Selegiline/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2441
Rapidly and completely metabolized to N-desmethyldeprenyl, l-methamphetamine, and l-amphetamine.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2442
Selegiline is extensively metabolized, presumably through cytochrome p450 mediated oxygenation, to form l-desmethylselegiline and l-methamphetamine, which is further metabolized to l-amphetamine. Selegiline also is metabolized in the lungs to l-desmethylselegiline and l-methamphetamine and in the kidneys to l-methamphetamine, but the degree of metabolism in these tissues is minimal compared with that in the liver. /Selegiline/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2441
... The pharmacokinetics of selegiline are highly variable. Following an oral dose of selegiline 10 mg, it is rapidly absorbed and metabolized to desmethylselegiline, levoamphetamine and levomethamphetamine. /Selegiline/
PMID:9260033 Mahmood I; Clin Pharmacokinet 33 (2): 91-102 (1997)
For more Metabolism/Metabolites (Complete) data for SELEGILINE HYDROCHLORIDE (6 total), please visit the HSDB record page.
Elimination: Selegiline: 39 (range, 16 to 69) hours. /Selegiline/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2442
The mean half-lives of the 3 active metabolites that were found in serum and urine following a single dose of selegiline are as follows: N-desmethyldeprenyl, 2 hours; l-amphetamine, 17.7 hours; l-methamphetamine, 20.5 hours. /Selegiline/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2442
The action of selegiline is thought to be related to its irreversible inhibition of monoamine oxidase type B (MAO B), the major form of the enzyme in the human brain. MAO B, which is involved in the oxidative deamination of dopamine in the brain, is inhibited when selegiline binds covalently and stoichiometrically to the isoalloxazine flavin adenine dinucleotide (FAD) at its active center. Administration of 10 mg of selegiline a day produces almost complete inhibition of MAO B in the brain. Selegiline becomes a nonselective inhibitor of all monamine oxidase (MAO) at higher doses, possibly at 20 to 40 mg a day. At these doses, tyramine-mediated hypertensive reaction with MAO A blockade ("cheese reactions") may occur. /Selegiline/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2442
Selegiline (or its metabolites) may also act through other mechanisms to increase dopaminergic activity, including interfering with dopamine reuptake at the synapse. /Selegiline/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2442
The mechanism of action of selegiline is complex and cannot be explained solely by its MAO-B inhibitory action. Pretreatment with selegiline can protect neurons against a variety of neurotoxins, such as 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP), 6-hydroxydopamine, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4), methyl-beta-acetoxyethyl-2-chloroethylamine (AF64A), and 5,6-dihydroxyserotonin, which damage dopaminergic, adrenergic, cholinergic, and sertoninergic neurons, respectively. Selegiline produces an amphetamine-like effect, enhances the release of dopamine, and blocks the reuptake of dopamine. It stimulates gene expression of L-aromatic amino acid decarboxylase, increases striatal phenylethylamine levels, and activates dopamine receptors. Selegiline reduces the production of oxidative radicals, up-regulates superoxide dismutase and catalase, and suppresses nonenzymatic and iron-catalyzed autooxidation of dopamine. Selegiline compensates for loss of target-derived trophic support, delays apoptosis in serum-deprived cells, and blocks apoptosis-related fall in the mitochondrial membrane potential. Most of the aforementioned properties occur independently of selegiline's efficacy to inhibit MAO-B. /Selegiline/
PMID:11813232 Ebadi M et al; J Neurosci Res 67 (3): 285-9 (2002)
Selegiline is a selective inhibitor of monoamine oxidase-B (MAO-B) at a dose of 10 mg/day and is given to patients with Parkinson's disease as an adjunct to levodopa therapy. By inhibiting MAO-B, selegiline increases the dopamine levels in the substantia nigra. Selegiline also blocks dopamine re-uptake from the synaptic cleft, thus increasing the dopamine concentrations in the brain. ... /Selegiline/
PMID:9260033 Mahmood I; Clin Pharmacokinet 33 (2): 91-102 (1997)
For more Mechanism of Action (Complete) data for SELEGILINE HYDROCHLORIDE (6 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
47
PharmaCompass offers a list of Selegiline Hydrochloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Selegiline Hydrochloride manufacturer or Selegiline Hydrochloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Selegiline Hydrochloride manufacturer or Selegiline Hydrochloride supplier.
PharmaCompass also assists you with knowing the Selegiline Hydrochloride API Price utilized in the formulation of products. Selegiline Hydrochloride API Price is not always fixed or binding as the Selegiline Hydrochloride Price is obtained through a variety of data sources. The Selegiline Hydrochloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Selegiline manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Selegiline, including repackagers and relabelers. The FDA regulates Selegiline manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Selegiline API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Selegiline manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Selegiline supplier is an individual or a company that provides Selegiline active pharmaceutical ingredient (API) or Selegiline finished formulations upon request. The Selegiline suppliers may include Selegiline API manufacturers, exporters, distributors and traders.
click here to find a list of Selegiline suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Selegiline DMF (Drug Master File) is a document detailing the whole manufacturing process of Selegiline active pharmaceutical ingredient (API) in detail. Different forms of Selegiline DMFs exist exist since differing nations have different regulations, such as Selegiline USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Selegiline DMF submitted to regulatory agencies in the US is known as a USDMF. Selegiline USDMF includes data on Selegiline's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Selegiline USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Selegiline suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Selegiline Drug Master File in Japan (Selegiline JDMF) empowers Selegiline API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Selegiline JDMF during the approval evaluation for pharmaceutical products. At the time of Selegiline JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Selegiline suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Selegiline Drug Master File in Korea (Selegiline KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Selegiline. The MFDS reviews the Selegiline KDMF as part of the drug registration process and uses the information provided in the Selegiline KDMF to evaluate the safety and efficacy of the drug.
After submitting a Selegiline KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Selegiline API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Selegiline suppliers with KDMF on PharmaCompass.
A Selegiline CEP of the European Pharmacopoeia monograph is often referred to as a Selegiline Certificate of Suitability (COS). The purpose of a Selegiline CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Selegiline EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Selegiline to their clients by showing that a Selegiline CEP has been issued for it. The manufacturer submits a Selegiline CEP (COS) as part of the market authorization procedure, and it takes on the role of a Selegiline CEP holder for the record. Additionally, the data presented in the Selegiline CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Selegiline DMF.
A Selegiline CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Selegiline CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Selegiline suppliers with CEP (COS) on PharmaCompass.
A Selegiline written confirmation (Selegiline WC) is an official document issued by a regulatory agency to a Selegiline manufacturer, verifying that the manufacturing facility of a Selegiline active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Selegiline APIs or Selegiline finished pharmaceutical products to another nation, regulatory agencies frequently require a Selegiline WC (written confirmation) as part of the regulatory process.
click here to find a list of Selegiline suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Selegiline as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Selegiline API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Selegiline as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Selegiline and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Selegiline NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Selegiline suppliers with NDC on PharmaCompass.
Selegiline Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Selegiline GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Selegiline GMP manufacturer or Selegiline GMP API supplier for your needs.
A Selegiline CoA (Certificate of Analysis) is a formal document that attests to Selegiline's compliance with Selegiline specifications and serves as a tool for batch-level quality control.
Selegiline CoA mostly includes findings from lab analyses of a specific batch. For each Selegiline CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Selegiline may be tested according to a variety of international standards, such as European Pharmacopoeia (Selegiline EP), Selegiline JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Selegiline USP).

