
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
South Africa
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
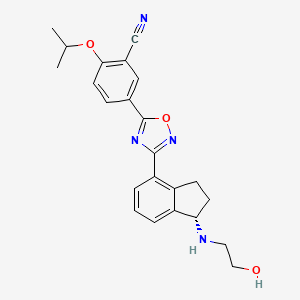

1. Rpc1063
1. 1306760-87-1
2. Rpc1063
3. Rpc-1063
4. Ozanimod (rpc1063)
5. (s)-5-(3-(1-((2-hydroxyethyl)amino)-2,3-dihydro-1h-inden-4-yl)-1,2,4-oxadiazol-5-yl)-2-isopropoxybenzonitrile
6. Z80293urpv
7. Zeposia
8. Unii-z80293urpv
9. Benzonitrile, 5-(3-((1s)-2,3-dihydro-1-((2-hydroxyethyl)amino)-1h-inden-4-yl)-1,2,4-oxadiazol-5-yl)-2-(1-methylethoxy)-
10. Ozanimod [inn]
11. 5-[3-[(1~{s})-1-(2-hydroxyethylamino)-2,3-dihydro-1~{h}-inden-4-yl]-1,2,4-oxadiazol-5-yl]-2-propan-2-yloxy-benzenecarbonitrile
12. Benzonitrile, 5-[3-[(1s)-2,3-dihydro-1-[(2-hydroxyethyl)amino]-1h-inden-4-yl]-1,2,4-oxadiazol-5-yl]-2-(1-methylethoxy)-
13. Ozanimod [usan:inn]
14. 5-[3-[(1s)-1-(2-hydroxyethylamino)-2,3-dihydro-1h-inden-4-yl]-1,2,4-oxadiazol-5-yl]-2-propan-2-yloxybenzonitrile
15. Rpc 1063
16. Ozanimod [usan]
17. Ozanimod; Rpc1063
18. Ozanimod (usan/inn)
19. Ozanimod [mi]
20. Ozanimod [who-dd]
21. Gtpl8709
22. Schembl2195490
23. Chembl3707247
24. Amy3373
25. Dtxsid501026488
26. Bcp16513
27. Ex-a1316
28. Rpc1063:rpc-1063
29. Bdbm50507186
30. Mfcd28386168
31. S7952
32. Akos026674086
33. Zinc116109867
34. Ccg-268695
35. Cs-5070
36. Db12612
37. Ac-29883
38. As-75063
39. Hy-12288
40. J3.612.016i
41. D10968
42. P14657
43. Q21098986
44. 5-{3-[(1s)-1-[(2-hydroxyethyl)amino]-2,3-dihydro-1h-inden-4-yl]-1,2,4-oxadiazol-5-yl}-2-(propan-2-yloxy)benzonitrile
45. Jeu
| Molecular Weight | 404.5 g/mol |
|---|---|
| Molecular Formula | C23H24N4O3 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 404.18484064 g/mol |
| Monoisotopic Mass | 404.18484064 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 609 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ozanimod is indicated for adults in the treatment of relapsing forms of MS, which may include relapsing-remitting disease, clinically isolated syndrome, and active secondary progressive MS.
FDA Label
Multiple sclerosis
- Zeposia is indicated for the treatment of adult patients with relapsing remitting multiple sclerosis (RRMS) with active disease as defined by clinical or imaging features.
Ulcerative colitis
- Zeposia is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response, or were intolerant to either conventional therapy or a biologic agent.
Ozanimod reduces circulating lymphocytes that cause the neuroinflammation associated with MS, reducing debilitating symptoms and, possibly, disease progression. During clinical trials, ozanimod reduced MS-associated brain volume loss in several regions. Ozanimod causes the sequestration of peripheral lymphocytes, reducing circulating lymphocytes in the gastrointestinal tract.
Sphingosine 1 Phosphate Receptor Modulators
Agents that affect the function of G-protein coupled SPHINGOSINE 1-PHOSPHATE RECEPTORS. Their binding to the receptors blocks lymphocyte migration and are often used as IMMUNOSUPPRESSANTS. (See all compounds classified as Sphingosine 1 Phosphate Receptor Modulators.)
L04AA38
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA38 - Ozanimod
Absorption
Ozanimod is absorbed in the gastrointestinal tract after oral administration. The Cmax of ozanimod is 0.244 ng/mL and is achieved at 6 to 8 hours after administration, reaching steady-state at about 102 hours after administration. The AUC is 4.46 ng*h/mL. Its delayed absorption reduces effects that may occur after the first dose, such as heart rate changes. The peak plasma concentration of ozanimod is low due to a high volume of distribution.
Route of Elimination
The kidneys are not a major source of elimination for ozanimod. After a 0.92 mg dose of radiolabeled ozanimod was administered, about 26% of the labeled drug was accounted for in the urine and 37 % in the feces, mainly in the form of inactive metabolites.
Volume of Distribution
The average volume of distribution of ozanimod is 5590L. Another reference mentions a volume of distribution ranging from 73-101 L/kg. This drug crosses the blood-brain barrier.
Clearance
The mean apparent oral clearance of ozanimod, according to prescribing information, is 192 L/h. Another reference indicates an oral clearance of 233 L/h.
Ozanimod has two major active metabolites CC112273 and CC1084037 and minor active metabolites such as RP101988, RP101075, and RP101509, which target the S1P1 and S1P5 receptors. The enzymes involved in the metabolism of ozanimod include ALDH/ADH, NAT-2, Monoamine Oxidase B, and AKR 1C1/1C2. After metabolism, ozanimod (6%), CC112273 (73%), and CC1084037 (15%) are accounted for in the circulation.
The half-life of ozanimod ranges from 17-21 hours.
Sphingosine1phosphate (S1P) is an important phospholipid that binds to various Gproteincoupled receptor subtypes, which can be identified as S1P15R. S1P and the receptors it binds to perform regular functions in the immune, cardiovascular, pulmonary, and nervous system. S1P can be expressed ubiquitously, playing an important role in regulating inflammation. S1P1R, S1P2R, and S1P3R receptors can be found in the cardiovascular, immune, and central nervous systems. S1P4R is found on lymphocytic and hematopoietic cells, while S1P5R expression is found only on the spleen (on natural killer cells) or in the central nervous system. Ozanimod is a selective modulator of S1P receptors and binds to S1P1R and S1P5R subtypes. The mechanism of action of ozanimod is not fully understood, but this drug likely reduces the migration of lymphocytes that usually aggravate the inflammation associated with MS.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
 Chunghwa provides cost-effective APIs & advanced intermediates with complete DMF or COS, ensuring quality & reliable production.
Chunghwa provides cost-effective APIs & advanced intermediates with complete DMF or COS, ensuring quality & reliable production.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37597
Submission : 2022-10-21
Status : Active
Type : II

NDC Package Code : 52076-6281
Start Marketing Date : 2024-05-16
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37015
Submission : 2022-03-31
Status : Active
Type : II

Date of Issue : 2024-02-12
Valid Till : 2027-02-11
Written Confirmation Number : WC-0407
Address of the Firm :

NDC Package Code : 70600-033
Start Marketing Date : 2022-03-24
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

| Available Reg Filing : CN, BR |

 Chunghwa provides cost-effective APIs & advanced intermediates with complete DMF or COS, ensuring quality & reliable production.
Chunghwa provides cost-effective APIs & advanced intermediates with complete DMF or COS, ensuring quality & reliable production.

NDC Package Code : 52076-6266
Start Marketing Date : 2020-12-11
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : Reviewed
Rev. Date : 2024-01-22
Pay. Date : 2023-11-17
DMF Number : 38668
Submission : 2023-11-23
Status : Active
Type : II



GDUFA
DMF Review : Reviewed
Rev. Date : 2023-11-10
Pay. Date : 2023-09-15
DMF Number : 38622
Submission : 2023-09-22
Status : Active
Type : II

NDC Package Code : 57741-3900
Start Marketing Date : 2025-02-26
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8531
Submission : 1990-04-17
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8462
Submission : 1990-02-22
Status : Inactive
Type : II


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Chunghwa provides cost-effective APIs & advanced intermediates with complete DMF or COS, ensuring quality & reliable production.
Chunghwa provides cost-effective APIs & advanced intermediates with complete DMF or COS, ensuring quality & reliable production.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37597
Submission : 2022-10-21
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37015
Submission : 2022-03-31
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8531
Submission : 1990-04-17
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2023-11-10
Pay. Date : 2023-09-15
DMF Number : 38622
Submission : 2023-09-22
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2024-01-22
Pay. Date : 2023-11-17
DMF Number : 38668
Submission : 2023-11-23
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8462
Submission : 1990-02-22
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Date of Issue : 2024-02-12
Valid Till : 2027-02-11
Written Confirmation Number : WC-0407
Address of the Firm : Sy. No. 205, 222 to 226, IDA Bonthapally, Bonthapally (Village), Gummadidala (Ma...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Details:
Wellosia (ozanimod) is a S1P modulator, small molecule drug candidate, which is indicated for the treatment of relapsing forms of multiple sclerosis & moderately to severely active ulcerative colitis.
Lead Product(s): Ozanimod,Inapplicable
Therapeutic Area: Neurology Brand Name: Wellosia
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 02, 2021
Lead Product(s) : Ozanimod,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Wellosia®; Prioritizing our Patients' Well-Being
Details : Wellosia (ozanimod) is a S1P modulator, small molecule drug candidate, which is indicated for the treatment of relapsing forms of multiple sclerosis & moderately to severely active ulcerative colitis.
Product Name : Wellosia
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 02, 2021
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Synthon will be responsible for obtaining final regulatory approval for its Ozanimod Capsules product (generic version of Zeposia), and thereafter, for the manufacture and supply of the product.
Lead Product(s): Ozanimod,Inapplicable
Therapeutic Area: Neurology Brand Name: Zeposia
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Zydus Lifesciences
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Licensing Agreement September 04, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ozanimod,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Zydus Lifesciences
Deal Size : Undisclosed
Deal Type : Licensing Agreement
Zydus and Synthon Entered into Exclusive Licensing and Supply Agreement for Ozanimod
Details : Synthon will be responsible for obtaining final regulatory approval for its Ozanimod Capsules product (generic version of Zeposia), and thereafter, for the manufacture and supply of the product.
Product Name : Zeposia
Product Type : Miscellaneous
Upfront Cash : Undisclosed
September 04, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Zeposia (ozanimod) is an oral, sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It is indicated for relapsing forms of multiple sclerosis.
Lead Product(s): Ozanimod,Inapplicable
Therapeutic Area: Neurology Brand Name: Zeposia
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 18, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ozanimod,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Zeposia Data Shows Long-Term Efficacy in Relapsing Multiple Sclerosis
Details : Zeposia (ozanimod) is an oral, sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It is indicated for relapsing forms of multiple sclerosis.
Product Name : Zeposia
Product Type : Miscellaneous
Upfront Cash : Inapplicable
September 18, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ozanimod is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Multiple Sclerosis, Relapsing-Remitting.
Lead Product(s): Ozanimod,Inapplicable
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 10, 2024

Details : Ozanimod is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Multiple Sclerosis, Relapsing-Remitting.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Zeposia (ozanimod) is an oral, sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It is being evaluated for the treatment of Crohn’s disease.
Lead Product(s): Ozanimod,Inapplicable
Therapeutic Area: Immunology Brand Name: Zeposia
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 28, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ozanimod,Inapplicable
Therapeutic Area : Immunology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Ascidian Collaborates with Roche for RNA Exon Editing Therapeutics
Details : Zeposia (ozanimod) is an oral, sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It is being evaluated for the treatment of Crohn’s disease.
Product Name : Zeposia
Product Type : Miscellaneous
Upfront Cash : Inapplicable
March 28, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Zeposia (ozanimod) is an oral, sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It is indicated for relapsing forms of multiple sclerosis.
Lead Product(s): Ozanimod,Inapplicable
Therapeutic Area: Neurology Brand Name: Zeposia
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Bristol Myers Squibb
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 29, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ozanimod,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Bristol Myers Squibb
Deal Size : Inapplicable
Deal Type : Inapplicable
Bristol Myers Releases Data Reinforcing Zeposia's Efficacy in MS
Details : Zeposia (ozanimod) is an oral, sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It is indicated for relapsing forms of multiple sclerosis.
Product Name : Zeposia
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 29, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ozanimod is a Other Small Molecule drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Colitis, Ulcerative.
Lead Product(s): Ozanimod,Inapplicable
Therapeutic Area: Immunology Brand Name: Undisclosed
Study Phase: Phase IVProduct Type: Miscellaneous
Sponsor: Bristol Myers Squibb
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ozanimod,Inapplicable
Therapeutic Area : Immunology
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : Bristol Myers Squibb
Deal Size : Inapplicable
Deal Type : Inapplicable
Ulcerative Colitis Leukocyte TRAfficking After Treatment With Zeposia: the ULTRAZ Study
Details : Ozanimod is a Other Small Molecule drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Colitis, Ulcerative.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
January 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ozanimod is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Colitis, Ulcerative.
Lead Product(s): Ozanimod,Inapplicable
Therapeutic Area: Immunology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 09, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ozanimod,Inapplicable
Therapeutic Area : Immunology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Ozanimod is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Colitis, Ulcerative.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 09, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Zeposia (ozanimod) is an oral, sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. Zeposia blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood.
Lead Product(s): Ozanimod,Inapplicable
Therapeutic Area: Neurology Brand Name: Zeposia
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 26, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ozanimod,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Zeposia (ozanimod) is an oral, sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. Zeposia blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in periphera...
Product Name : Zeposia
Product Type : Miscellaneous
Upfront Cash : Inapplicable
October 26, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Zeposia (ozanimod) is an oral, sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5 also maintains disease control even in the event of temporary treatment interruption for up to eight weeks.
Lead Product(s): Ozanimod,Inapplicable
Therapeutic Area: Immunology Brand Name: Zeposia
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 24, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Ozanimod,Inapplicable
Therapeutic Area : Immunology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Zeposia (ozanimod) is an oral, sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5 also maintains disease control even in the event of temporary treatment interruption for up to eight weeks.
Product Name : Zeposia
Product Type : Miscellaneous
Upfront Cash : Inapplicable
October 24, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results](S)-1-amino-2,3-dihydro-1H-indene-4-carbonitrile h...
CAS Number : 1306763-57-4
End Use API : Ozanimod
About The Company : Aarti Pharmalabs is generic APIs & Intermediates manufacturing company & small molecule drug substance CDMO and the largest Indian manufacturer of Xanthine Deri...
(S)-1-amino-2,3-dihydro-1H-indene-4-carbonitrile h...
CAS Number : 1306763-57-4
End Use API : Ozanimod
About The Company : Sichuan Taienkang Pharmaceutical Co., Ltd., established in 2020, is a subsidiary of Guangdong Taienkang Pharmaceutical Co., Ltd. The company specializes in the ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : ZEPOSIA
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 0.23MG BASE
Approval Date : 2020-03-25
Application Number : 209899
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : ZEPOSIA
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 0.46MG BASE
Approval Date : 2020-03-25
Application Number : 209899
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : ZEPOSIA
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 0.92MG BASE
Approval Date : 2020-03-25
Application Number : 209899
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : OZANIMOD
Dosage Form : CAPSULE
Dosage Strength : 0.46MG
Approval Date :
Application Number : 219236
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : OZANIMOD
Dosage Form : CAPSULE
Dosage Strength : 0.23MG
Approval Date :
Application Number : 219236
RX/OTC/DISCN :
RLD :
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Zeposia
Dosage Form : Hard Capsule
Dosage Strength : 0.23mg
Packaging :
Approval Date : 11/08/2020
Application Number : 67046
Regulatory Info : Allowed
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Zeposia
Dosage Form : Hard Capsule
Dosage Strength : 0.46mg
Packaging :
Approval Date : 11/08/2020
Application Number : 67046
Regulatory Info : Allowed
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Zeposia
Dosage Form : Hard Capsule
Dosage Strength : 0.92mg
Packaging :
Approval Date : 11/08/2020
Application Number : 67046
Regulatory Info : Allowed
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Marketed
Registration Country : Norway
Brand Name : Zeposia
Dosage Form : Capsule
Dosage Strength : 0.92mg
Packaging :
Approval Date :
Application Number :
Regulatory Info : Marketed
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Estonia
Brand Name : Zeposia
Dosage Form : Capsule
Dosage Strength : 0.23mg
Packaging :
Approval Date :
Application Number :
Regulatory Info : Prescription
Registration Country : Estonia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Estonia
Brand Name : Zeposia
Dosage Form : Capsule
Dosage Strength : 0.92mg
Packaging :
Approval Date :
Application Number :
Regulatory Info : Prescription
Registration Country : Estonia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Authorized
Registration Country : Spain
Brand Name : Zeposia
Dosage Form : Hard Capsule
Dosage Strength : 0.23MG; 0.46 MG
Packaging :
Approval Date : 25-06-2020
Application Number : 1201442001
Regulatory Info : Authorized
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Authorized
Registration Country : Spain
Brand Name : Zeposia
Dosage Form : Hard Capsule
Dosage Strength : 0.92MG
Packaging :
Approval Date : 25-06-2020
Application Number : 1201442002
Regulatory Info : Authorized
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Denmark
Brand Name : Zeposia
Dosage Form : Hard Capsules
Dosage Strength : 0.92mg
Packaging :
Approval Date : 20-05-2020
Application Number : 28106239319
Regulatory Info : Prescription
Registration Country : Denmark

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Zeposia
Dosage Form : Capsule
Dosage Strength : 0.23mg, 0.46mg
Packaging :
Approval Date : 20-05-2020
Application Number : 2.02E+13
Regulatory Info : Approved
Registration Country : Sweden

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
04 Sep 2025
// PR NEWSWIRE
https://www.prnewswire.com/news-releases/zydus-and-synthon-entered-into-exclusive-licensing-and-supply-agreement-for-ozanimod-capsules-generic-version-of-zeposia-for-the-us-market-302546536.html

22 Jul 2025
// FDA
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=219236

14 Oct 2024
// EXPRESSPHARMA
https://www.expresspharma.in/s1p-receptor-modulators-face-uncertain-future-in-crohns-disease-amid-trial-setbacks/

18 Sep 2024
// BUSINESSWIRE

05 Jun 2024
// FDA
https://www.pharmacompass.com/pdf/news/fda-confirms-para-iv-patent-litigation-for-ozanimod-hcl-zeposia-capsules-92708.pdf

29 Mar 2024
// REUTERS
https://www.reuters.com/business/healthcare-pharmaceuticals/bristol-myers-bowel-disease-drug-fails-meet-main-goal-late-stage-study-2024-03-28/
Global Sales Information

Company : Celgene/BMS
Ozanimod Hydrochloride
Drug Cost (USD) : 62,945,499
Year : 2023
Prescribers : 1034
Prescriptions : 6180

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Celgene/BMS
Ozanimod Hydrochloride
Drug Cost (USD) : 37,767,664
Year : 2022
Prescribers : 655
Prescriptions : 3962

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Celgene/BMS
Ozanimod Hydrochloride
Drug Cost (USD) : 19,598,158
Year : 2021
Prescribers : 406
Prescriptions : 2193

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Celgene/BMS
Ozanimod Hydrochloride
Drug Cost (USD) : 2,103,131
Year : 2020
Prescribers : 104
Prescriptions : 243

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Main Therapeutic Indication : Neurology
Currency : USD
2020 Revenue in Millions : 12
2019 Revenue in Millions : 0
Growth (%) : New Launch

Main Therapeutic Indication : Neurology
Currency : USD
2021 Revenue in Millions : 134
2020 Revenue in Millions : 12
Growth (%) : 1,017

Main Therapeutic Indication : Neurology
Currency : USD
2022 Revenue in Millions : 250
2021 Revenue in Millions : 134
Growth (%) : 87

Main Therapeutic Indication : Neurology
Currency : USD
2023 Revenue in Millions : 434
2022 Revenue in Millions : 250
Growth (%) : 74

Main Therapeutic Indication : Neurology
Currency : USD
2024 Revenue in Millions : 566
2023 Revenue in Millions : 434
Growth (%) : 30

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
15 Sep 2025
Reply
06 Jun 2025
Reply
28 Nov 2024
Reply
26 Jul 2022
Reply
26 Sep 2020
Reply
12 Jun 2020
Reply
10 Jun 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

CAS Number : 1618636-37-5
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : O0049.01

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
100
PharmaCompass offers a list of Ozanimod API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ozanimod manufacturer or Ozanimod supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ozanimod manufacturer or Ozanimod supplier.
PharmaCompass also assists you with knowing the Ozanimod API Price utilized in the formulation of products. Ozanimod API Price is not always fixed or binding as the Ozanimod Price is obtained through a variety of data sources. The Ozanimod Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ozanimod Hydrochloride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ozanimod Hydrochloride, including repackagers and relabelers. The FDA regulates Ozanimod Hydrochloride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ozanimod Hydrochloride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ozanimod Hydrochloride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ozanimod Hydrochloride supplier is an individual or a company that provides Ozanimod Hydrochloride active pharmaceutical ingredient (API) or Ozanimod Hydrochloride finished formulations upon request. The Ozanimod Hydrochloride suppliers may include Ozanimod Hydrochloride API manufacturers, exporters, distributors and traders.
click here to find a list of Ozanimod Hydrochloride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ozanimod Hydrochloride DMF (Drug Master File) is a document detailing the whole manufacturing process of Ozanimod Hydrochloride active pharmaceutical ingredient (API) in detail. Different forms of Ozanimod Hydrochloride DMFs exist exist since differing nations have different regulations, such as Ozanimod Hydrochloride USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ozanimod Hydrochloride DMF submitted to regulatory agencies in the US is known as a USDMF. Ozanimod Hydrochloride USDMF includes data on Ozanimod Hydrochloride's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ozanimod Hydrochloride USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ozanimod Hydrochloride suppliers with USDMF on PharmaCompass.
A Ozanimod Hydrochloride written confirmation (Ozanimod Hydrochloride WC) is an official document issued by a regulatory agency to a Ozanimod Hydrochloride manufacturer, verifying that the manufacturing facility of a Ozanimod Hydrochloride active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Ozanimod Hydrochloride APIs or Ozanimod Hydrochloride finished pharmaceutical products to another nation, regulatory agencies frequently require a Ozanimod Hydrochloride WC (written confirmation) as part of the regulatory process.
click here to find a list of Ozanimod Hydrochloride suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ozanimod Hydrochloride as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ozanimod Hydrochloride API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ozanimod Hydrochloride as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ozanimod Hydrochloride and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ozanimod Hydrochloride NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ozanimod Hydrochloride suppliers with NDC on PharmaCompass.
Ozanimod Hydrochloride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ozanimod Hydrochloride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ozanimod Hydrochloride GMP manufacturer or Ozanimod Hydrochloride GMP API supplier for your needs.
A Ozanimod Hydrochloride CoA (Certificate of Analysis) is a formal document that attests to Ozanimod Hydrochloride's compliance with Ozanimod Hydrochloride specifications and serves as a tool for batch-level quality control.
Ozanimod Hydrochloride CoA mostly includes findings from lab analyses of a specific batch. For each Ozanimod Hydrochloride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ozanimod Hydrochloride may be tested according to a variety of international standards, such as European Pharmacopoeia (Ozanimod Hydrochloride EP), Ozanimod Hydrochloride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ozanimod Hydrochloride USP).

