
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
South Africa
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
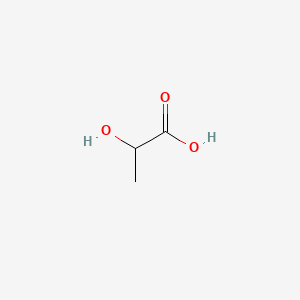

1. 2 Hydroxypropanoic Acid
2. 2 Hydroxypropionic Acid
3. 2-hydroxypropanoic Acid
4. 2-hydroxypropionic Acid
5. Ammonium Lactate
6. D Lactic Acid
7. D-lactic Acid
8. L Lactic Acid
9. L-lactic Acid
10. Lactate
11. Lactate, Ammonium
12. Propanoic Acid, 2-hydroxy-, (2r)-
13. Propanoic Acid, 2-hydroxy-, (2s)-
14. Sarcolactic Acid
1. 2-hydroxypropanoic Acid
2. Dl-lactic Acid
3. 50-21-5
4. 2-hydroxypropionic Acid
5. Milk Acid
6. Lactate
7. Tonsillosan
8. Racemic Lactic Acid
9. Ordinary Lactic Acid
10. Polylactic Acid
11. Ethylidenelactic Acid
12. Lactovagan
13. Acidum Lacticum
14. 26100-51-6
15. Lactic Acid, Dl-
16. Propanoic Acid, 2-hydroxy-
17. Kyselina Mlecna
18. Lacticum Acidum
19. Dl-milchsaeure
20. (+/-)-lactic Acid
21. 598-82-3
22. 1-hydroxyethanecarboxylic Acid
23. Aethylidenmilchsaeure
24. Alpha-hydroxypropionic Acid
25. (rs)-2-hydroxypropionsaeure
26. Fema No. 2611
27. Kyselina 2-hydroxypropanova
28. Propionic Acid, 2-hydroxy-
29. Purac Fcc 80
30. Purac Fcc 88
31. Ccris 2951
32. Hsdb 800
33. (+-)-2-hydroxypropanoic Acid
34. Lurex
35. Lactic Acid, Tech Grade
36. Propanoic Acid, Hydroxy-
37. Sy-83
38. Dl- Lactic Acid
39. Nsc 367919
40. 2-hydroxypropionicacid
41. Ai3-03130
42. Mfcd00004520
43. Hipure 88
44. Nsc-367919
45. .alpha.-hydroxypropanoic Acid
46. .alpha.-hydroxypropionic Acid
47. (r)-2-hydroxy-propionic Acid;h-d-lac-oh
48. Ins No.270
49. E 270
50. (+/-)-2-hydroxypropanoic Acid
51. Chebi:78320
52. Ins-270
53. Poly(l-lactide)
54. 3b8d35y7s4
55. Lactic Acid Usp
56. Ncgc00090972-01
57. Lactic Acid, 1.0n Standardized Solution
58. 2-hydroxy-propionic Acid
59. Lactic Acid (natural)
60. E-270
61. Dsstox_cid_3192
62. Alpha-hydroxypropanoic Acid
63. C01432
64. Dsstox_rid_76915
65. Dsstox_gsid_23192
66. Milchsaure [german]
67. Lactic Acid [jan]
68. Milchsaure
69. Fema Number 2611
70. Kyselina Mlecna [czech]
71. 163894-00-6
72. Cheongin Samrakhan
73. Cas-50-21-5
74. Cheongin Haewoohwan
75. Cheongin Haejanghwan
76. Kyselina 2-hydroxypropanova [czech]
77. Einecs 200-018-0
78. Einecs 209-954-4
79. Lactic Acid [usp:jan]
80. Epa Pesticide Chemical Code 128929
81. Brn 5238667
82. Lactasol
83. 1-hydroxyethane 1-carboxylic Acid
84. Biolac
85. Unii-3b8d35y7s4
86. 2-hydroxy-2-methylacetic Acid
87. Lactide Polymer
88. Chem-cast
89. L- Lactic Acid
90. Dl-polylactic Acid
91. Lactate (tn)
92. Lactic Acid,buffered
93. 4b5w
94. Propanoic Acid, (+-)
95. Dl-lactic Acid, Racemic
96. (.+/-.)-lactic Acid
97. Ec 200-018-0
98. Lactic Acid (7ci,8ci)
99. Lactic Acid (jp17/usp)
100. Lactic Acid, 85%, Fcc
101. Lactic Acid, Racemic, Usp
102. Nciopen2_000884
103. Lactic Acid (+-)
104. Dl-lactic Acid [mi]
105. Lactic Acid, Unspecified Form
106. Lactic Acid [who-ip]
107. (rs)-2-hydroxypropanoic Acid
108. Lactic Acid (fragrance Grade)
109. Lacticum Acidum [hpus]
110. Dl-lactic Acid (90per Cent)
111. Chembl1200559
112. Dtxsid7023192
113. Lactic Acid, Natural, >=85%
114. Bdbm23233
115. L-lactic Acid Or Dl-lactic Acid
116. Lactic Acid, 85 Percent, Fcc
117. Lactic Acid, Dl- [ii]
118. Dl-lactic Acid, ~90% (t)
119. Dl-lactic Acid, Ar, >=88%
120. Dl-lactic Acid, Lr, >=88%
121. Dl- Lactic Acid [who-dd]
122. Lactic Acid, 10 Percent Solution
123. Hy-b2227
124. Propanoic Acid, 2-hydroxy- (9ci)
125. Tox21_111049
126. Tox21_202455
127. Tox21_303616
128. Bbl027466
129. Nsc367919
130. Stl282744
131. Akos000118855
132. Akos017278364
133. Tox21_111049_1
134. Acidum Lacticum [who-ip Latin]
135. Am87208
136. Db04398
137. Sb44647
138. Sb44652
139. Dl-lactic Acid, 85 % (w/w), Syrup
140. Propanoic Acid,2-hydroxy-,(.+/-.)-
141. 2-hydroxypropionic Acid, Dl-lactic Acid
142. Ncgc00090972-02
143. Ncgc00090972-03
144. Ncgc00257515-01
145. Ncgc00260004-01
146. 26811-96-1
147. Lactic Acid, 85 Percent, Reagent, Acs
148. Db-071134
149. Lactic Acid 100 Microg/ml In Acetonitrile
150. Cs-0021601
151. Ft-0624390
152. Ft-0625477
153. Ft-0627927
154. Ft-0696525
155. Ft-0774042
156. L0226
157. Lactic Acid Solution, Acs Reagent, >=85%
158. Lactic Acid Solution, Usp, 88.0-92.0%
159. Lactic Acid Solution, P.a., 84.5-85.5%
160. Lactic Acid, Meets Usp Testing Specifications
161. D00111
162. F71201
163. A877374
164. Dl-lactic Acid, Saj First Grade, 85.0-92.0%
165. Q161249
166. Dl-lactic Acid, Jis Special Grade, 85.0-92.0%
167. Lactic Acid Solution, Vetec(tm) Reagent Grade, 85%
168. F2191-0200
169. Bc10f553-5d5d-4388-bb74-378ed4e24908
170. Lactic Acid, United States Pharmacopeia (usp) Reference Standard
171. Lactic Acid, Pharmaceutical Secondary Standard; Certified Reference Material
172. Dl-lactic Acid 90%, Synthetic, Meets The Analytical Specifications Of Ph. Eur.
173. 152-36-3
| Molecular Weight | 90.08 g/mol |
|---|---|
| Molecular Formula | C3H6O3 |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 90.031694049 g/mol |
| Monoisotopic Mass | 90.031694049 g/mol |
| Topological Polar Surface Area | 57.5 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 59.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Lactic acid is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=lactic+acid&Search=Search
(VET): Has been used as a caustic, and in dilute solutions to irrigate tissues; as an intestinal antiseptic and antiferment.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 990
A 10% solution is used as a bactericidal agent on the skin of neonates. ... A 16.7% solution in flexible collodion is used to remove warts and small cutaneous tumors.
Troy, D.B. (Ed); Remmington The Science and Practice of Pharmacy. 21 st Edition. Lippincott Williams & Williams, Philadelphia, PA 2005, p. 1087
Acidulant
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 990
For use as an alkalinizing agent.
Lactic acid produces a metabolic alkalinizing effect.
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AD - Organic acids
G01AD01 - Lactic acid
L-lactic acid occurs in small quantities in the blood and muscle fluid of humans and animals; the concentration of lactic acid in these fluids increases after vigorous activity. L-lactic acid is also present in the liver, kidneys, thymus gland, human amniotic fluid, and other organs and body fluids.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology, 17 (Suppl.1): 1-203 (1998)
A primed infusion study was performed /in humans/ using radioactive L-lactic acid. The virtual volume of distribution of lactate was 49.4% of body weight. The lactate pool size and turnover time were estimated as 0.029 g/kg and 18.4 min, respectively.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology, 17 (Suppl.1): 1-203 (1998)
In the body, lactate is distributed equivalently to, or slightly less than, total body water. It diffuses readily across cell membranes, primarily by passive transport; under certain conditions, the distribution could be uneven or the lactate pool could consist of several smaller pools with differing rate constants.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology, 17 (Suppl.1): 1-203 (1998)
The percutaneous absorption of topically applied 5% [14C]-lactic acid in an oil-in-water cream was measured using rats. After 3 days, 50% of the applied lactic acid had penetrated the skin.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology, 17 (Suppl.1): 1-203 (1998)
For more Absorption, Distribution and Excretion (Complete) data for LACTIC ACID (6 total), please visit the HSDB record page.
... Propylene glycol ... is oxidized to lactic acid or pyruvic acid by two pathways. These two metabolites are then used by the body as sources of energy either by oxidation through the tricarboxylic acid cycle or by generation of glycogen through the glycolytic pathway.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 31
Lactic acid diffuses through muscle tissue and is transported to the liver in the bloodstream. In the liver, it is converted to glucose by gluconeogenesis. Lactic acid can also be further catabolized in the lactic acid cycle (also known as the Cori cycle).
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additives Ser 48: Lactic acid (2002). Available from, as of April 19, 2006: https://www.inchem.org/documents/jecfa/jecmono/v48je16.htm
L-lactic acid is a normal metabolic intermediate produced by most mammalian cells and other organisms, such as bacteria; it is metabolized in preference to D-lactic acid in man, dogs, and rats. Lactic acid is converted to pyruvic acid by lactic acid dehydrogenase.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology, 17 (Suppl.1): 1-203 (1998)
In animals, lactate that is generated by anaerobic metabolism can be transported to other more aerobic tissues, such as the liver, where it can be reconverted to pyruvate. The pyruvate can then be further metabolized, reconverted to carbohydrate material as free glucose, or stored as glycogen.
Cosmetic Ingredient Review Expert Panel; International Journal of Toxicology, 17 (Suppl.1): 1-203 (1998)
For more Metabolism/Metabolites (Complete) data for LACTIC ACID (8 total), please visit the HSDB record page.
Lactate ions are metabolized ultimately to carbon dioxide and water, which requires the consumption of hydrogen cations.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
68
PharmaCompass offers a list of Lactic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lactic Acid manufacturer or Lactic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lactic Acid manufacturer or Lactic Acid supplier.
PharmaCompass also assists you with knowing the Lactic Acid API Price utilized in the formulation of products. Lactic Acid API Price is not always fixed or binding as the Lactic Acid Price is obtained through a variety of data sources. The Lactic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A lactasol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of lactasol, including repackagers and relabelers. The FDA regulates lactasol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. lactasol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of lactasol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A lactasol supplier is an individual or a company that provides lactasol active pharmaceutical ingredient (API) or lactasol finished formulations upon request. The lactasol suppliers may include lactasol API manufacturers, exporters, distributors and traders.
click here to find a list of lactasol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A lactasol DMF (Drug Master File) is a document detailing the whole manufacturing process of lactasol active pharmaceutical ingredient (API) in detail. Different forms of lactasol DMFs exist exist since differing nations have different regulations, such as lactasol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A lactasol DMF submitted to regulatory agencies in the US is known as a USDMF. lactasol USDMF includes data on lactasol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The lactasol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of lactasol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The lactasol Drug Master File in Japan (lactasol JDMF) empowers lactasol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the lactasol JDMF during the approval evaluation for pharmaceutical products. At the time of lactasol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of lactasol suppliers with JDMF on PharmaCompass.
lactasol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of lactasol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right lactasol GMP manufacturer or lactasol GMP API supplier for your needs.
A lactasol CoA (Certificate of Analysis) is a formal document that attests to lactasol's compliance with lactasol specifications and serves as a tool for batch-level quality control.
lactasol CoA mostly includes findings from lab analyses of a specific batch. For each lactasol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
lactasol may be tested according to a variety of international standards, such as European Pharmacopoeia (lactasol EP), lactasol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (lactasol USP).

