
Synopsis
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book
0
Australia
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
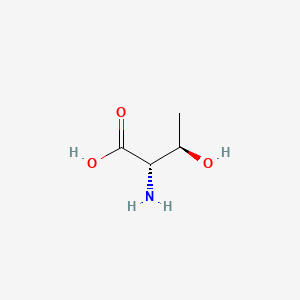

1. L Threonine
2. Threonine
1. Threonine
2. 72-19-5
3. (2s,3r)-2-amino-3-hydroxybutanoic Acid
4. L-(-)-threonine
5. Threonin
6. (s)-threonine
7. Dl-threonine
8. H-thr-oh
9. 2-amino-3-hydroxybutyric Acid
10. 80-68-2
11. Threonine, L-
12. Threonine (van)
13. Threoninum [latin]
14. Treonina [spanish]
15. (2s,3r)-2-amino-3-hydroxybutyric Acid
16. Threonine [usan:inn]
17. Thre
18. L-threonin
19. L-thr
20. Threonine, Dl-
21. (2s)-threonine
22. L-alpha-amino-beta-hydroxybutyric Acid
23. (2s,3r)-(-)-threonine
24. 2-amino-3-hydroxybutanoic Acid, (r-(r*,s*))-
25. Butanoic Acid, 2-amino-3-hydroxy-, (r-(r*,s*))-
26. L-2-amino-3-hydroxybutyric Acid
27. Mfcd00064270
28. Tfm6du5s6a
29. Fema No. 4710
30. Chebi:16857
31. [r-(r*,s*)]-2-amino-3-hydroxybutanoic Acid
32. Nsc-16589
33. Nsc-46701
34. 2zd004190s
35. H-dl-thr-oh
36. Threonine (l)
37. 6028-28-0
38. (2s,3r)-rel-2-amino-3-hydroxybutanoic Acid
39. (r-(r*,s*))-2-amino-3-hydroxybutanoic Acid
40. Dsstox_cid_26412
41. Dsstox_rid_81591
42. Dsstox_gsid_46412
43. Thr
44. Threonine #
45. Threoninum
46. Treonina
47. Allothreonine, D-
48. Cas-72-19-5
49. (r-(r*,s*))-2-amino-3-hydroxybutanoate
50. [r-(r*,s*)]-2-amino-3-hydroxybutanoate
51. L-(u-14c)threonine
52. Einecs 200-774-1
53. Nsc 16589
54. Nsc 46701
55. Nsc46701
56. Unii-2zd004190s
57. Hsdb 7797
58. L-threonine;
59. Nsc-206292
60. Ncgc00164520-01
61. Ncgc00164520-02
62. Threonine (usp)
63. L-threonine,(s)
64. (+/-)-threonine
65. L-thr-oh
66. L-threonine (9ci)
67. Threonine, Labeled With Carbon-14, L-
68. Threonine [inn]
69. L-threonine (jp17)
70. Threonine [ii]
71. Threonine [mi]
72. Threonine (l-threonine)
73. Threonine [hsdb]
74. Threonine [inci]
75. Threonine [usan]
76. Unii-tfm6du5s6a
77. Threonine [vandf]
78. Threonine, L- (8ci)
79. 2-amino-3-hydroxybutyrate
80. Bmse000049
81. Bmse000810
82. Bmse000859
83. L-threonine [fcc]
84. L-threonine [jan]
85. Threonine [mart.]
86. L-threonine (h-thr-oh)
87. 2-amino-3-hydroxybutanoate
88. Schembl1480
89. Threonine [who-dd]
90. L-2-amino-3-hydroxybutyrate
91. L-threonine [usp-rs]
92. L-threonine Non-animal Source
93. Ccris 8603
94. Chembl291747
95. Gtpl4785
96. Dtxsid2046412
97. Threonine [ep Monograph]
98. Dtxsid70893087
99. Threonine [usp Monograph]
100. L-alpha-amino-beta-hydroxybutyrate
101. L-threonine, Cell Culture Reagent
102. Pharmakon1600-01301008
103. Zinc895103
104. Hy-n0658
105. Einecs 201-300-6
106. Tox21_112154
107. Ac7878
108. Nsc760118
109. S4951
110. (2s,3r)-2-amino-3-hydroxybutyrate
111. Akos006240505
112. Akos015840277
113. Tox21_112154_1
114. Ccg-214540
115. Cs-w020046
116. Db00156
117. Nsc 206292
118. Nsc-760118
119. 7013-32-3
120. Ac-11296
121. As-12789
122. L-threonine, Bioxtra, >=99.5% (nt)
123. L-threonine, P.a., 99.0-101.0%
124. Ai3-18477
125. Db-029984
126. (2s,3r)-rel-2-amino-3-hydroxybutanoicacid
127. [r-(r*,s*)]-2-amino-3-hydroxy-butanoate
128. Am20100684
129. T0230
130. L-threonine, Reagent Grade, >=98% (hplc)
131. 72t195
132. C00188
133. D00041
134. M02962
135. [r-(r*,s*)]-2-amino-3-hydroxy-butanoic Acid
136. L-threonine, Vetec(tm) Reagent Grade, >=98%
137. Q186521
138. 3dd2e9ad-db9a-460e-8a6b-c01b0f67ac4e
139. Q-201331
140. Lysine Hydrochloride Impurity C [ep Impurity]
141. 48: Pn: Wo2004076659 Figure: 7 Claimed Sequence
142. F8881-8883
143. L-threonine, Certified Reference Material, Tracecert(r)
144. Z1259341114
145. L-threonine, European Pharmacopoeia (ep) Reference Standard
146. L-threonine, United States Pharmacopeia (usp) Reference Standard
147. L-threonine, Pharmaceutical Secondary Standard; Certified Reference Material
148. L-threonine, From Non-animal Source, Meets Ep, Jp, Usp Testing Specifications, Suitable For Cell Culture, 99.0-101.0%
149. L-threonine, Pharmagrade, Ajinomoto, Ep, Jp, Usp, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production, Suitable For Cell Culture
| Molecular Weight | 119.12 g/mol |
|---|---|
| Molecular Formula | C4H9NO3 |
| XLogP3 | -2.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 119.058243149 g/mol |
| Monoisotopic Mass | 119.058243149 g/mol |
| Topological Polar Surface Area | 83.6 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 93.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
L-threonine has been used clinically with the aim of increasing glycine concentrations in the cerebral spinal fluid of patients with spasticity. When given in amounts of 4.5 to 6.0 g/day for 14 days, no adverse clinical effects were noted in such patients.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 731, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
/Experimental Therapy/ To determine whether the naturally occurring amino acid threonine, a potential precursor for glycine biosynthesis in the spinal cord, has an effect on spasticity in multiple sclerosis, 26 ambulatory patients were entered into a randomized crossover trial. Threonine administered at a total daily dose of 7.5 g reduced signs of spasticity on clinical examination, although no symptomatic improvement could be detected by the examining physician or the patient. In contrast to the side effects of sedation and increased motor weakness associated with antispasticity drugs commonly used for the treatment of multiple sclerosis, no side effects or toxic effects of threonine were identified...
PMID:1520082 Hauser SL et al; Arch Neurol 49 (9): 923-6 (1992).
/Experimental Therapy/ ... 4.5 and 6.0 g/day of L-threonine /was administered/ to 18 patients with familial spastic paraparesis (FSP) according to a double-blind, crossover protocol. ... L-threonine significantly suppressed the signs of spasticity even though the benefits were not clinically valuable.
PMID:1742749 Growdon JH et al; Clin Neuropharmacol 14 (5): 403-12 (1991).
/Experimental Therapy/ A randomized, double-blind, placebo-controlled trial was carried out in 22 patients with hypostatic leg ulceration. Patients were treated topically with either a cream containing the amino acids l-cysteine, glycine and dl-threonine or the cream base alone (placebo). Most patients had their leg ulcers treated and dressed 3-times per week for 12 weeks. ... The degree of healing and decrease of pain were significantly better in the group of patients receiving the amino acid combination. It would appear from this study that l-cysteine, glycine and dl-threonine in combination are of value in promoting would healing in hypostatic leg ulceration.
PMID:3933019 Harvey SG et al; Pharmatherapeutica 4 (4): 227-30 (1985).
... In this placebo-controlled crossover study, the effect of supplemental oral threonine (THR) on the plasma amino acid concentrations of 12 patients with hyperphenylalaninemia was investigated. Before starting the first treatment period of this cross-over study, the patients were randomly assigned to one of two groups supplemented either with approximately 50 mg THR/kg per day or with a similar amount of maltodextrin as placebo. After a feeding period of 8 weeks and a wash-out period of 8 weeks, the supplements were crossed over and the study continued for an additional 8 weeks. Blood was obtained at the start and the end of each supplementation period. Dietary THR supplementation of approximately 50 mg/kg per day resulted in a significant decrease of plasma phenylalanine (PHE) levels ( P = 0.0234). There was a close positive correlation between plasma and urinary PHE concentrations ( P < 0.001) indicating that the lower plasma PHE levels in the THR supplemented patients were not caused by higher urinary excretion of PHE. CONCLUSIONS: The data of the present study show that oral THR supplementation has a clear plasma-PHE-reducing effect but they do not allow any conclusion about the mechanisms responsible for the observed effect. Although it seems attractive on the basis of the present data to use THR supplementation in patients with hyperphenylalaninemia, the mechanism of the observed effect should be clarified before introduction of such a treatment in these patients.
PMID:12499992 Sanjurjo P et al; J Pediatr Gastroenterol Nutr 36 (1): 23-6 (2003).
A two center, double-blind, placebo-controlled treatment trial with oral branched chain amino acids (BCAA) (L-leucine 12 g, L-isoleucine 8 g, and L-valine 6.4 g daily) or L-threonine (4 g daily) with pyridoxal phosphate (160 mg daily) /was conducted/ for six months in patients with amyotrophic lateral sclerosis (ALS). ... The amino acids were well tolerated. The results of our study failed to show a beneficial effect of BCAA or L-threonine treatment for six months on the disease course in ALS. The higher rate of loss of pulmonary function in patients treated with BCAA or L-threonine may have been due to chance, but an adverse effect of these amino acids cannot be ruled out.
PMID:8909433 Tandan R et al; Neurology 47 (5): 1220-6 (1996).
The threonine content of most of the infant formulas currently on the market is approximately 20% higher than the threonine concentration in human milk. Due to this high threonine content the plasma threonine concentrations are up to twice as high in premature infants fed these formulas than in infants fed human milk. Increasing the threonine in plasma leads to increasing brain glycine and thereby affects the neurotransmitter balance in the brain. This may have consequences for brain development during early postnatal life. Therefore, excessive threonine intake during infant feeding should be avoided.
PMID:9853925 Boehm G et al; Pediatr Res 44 (6): 900-6 (1998).
L-Threonine makes up collagen, elastin, and enamel protein. It aids proper fat metabolism in the liver, helps the digestive and intestinal tracts function more smoothly, and assists in metabolism and assimilation.
L-Threonine is an essential amino acid that helps to maintain the proper protein balance in the body. It is important for the formation of collagen, elastin, and tooth enamel, and aids liver and lipotropic function when combined with aspartic acid and methionine.
Although the free amino acids dissolved in the body fluids are only a very small proportion of the body's total mass of amino acids, they are very important for the nutritional and metabolic control of the body's proteins. ... Although the plasma compartment is most easily sampled, the concentration of most amino acids is higher in tissue intracellular pools. Typically, large neutral amino acids, such as leucine and phenylalanine, are essentially in equilibrium with the plasma. Others, notably glutamine, glutamic acid, and glycine, are 10- to 50-fold more concentrated in the intracellular pool. Dietary variations or pathological conditions can result in substantial changes in the concentrations of the individual free amino acids in both the plasma and tissue pools. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 596, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
After ingestion, proteins are denatured by the acid in the stomach, where they are also cleaved into smaller peptides by the enzyme pepsin, which is activated by the increase in stomach acidity that occurs on feeding. The proteins and peptides then pass into the small intestine, where the peptide bonds are hydrolyzed by a variety of enzymes. These bond-specific enzymes originate in the pancreas and include trypsin, chymotrypsins, elastase, and carboxypeptidases. The resultant mixture of free amino acids and small peptides is then transported into the mucosal cells by a number of carrier systems for specific amino acids and for di- and tri-peptides, each specific for a limited range of peptide substrates. After intracellular hydrolysis of the absorbed peptides, the free amino acids are then secreted into the portal blood by other specific carrier systems in the mucosal cell or are further metabolized within the cell itself. Absorbed amino acids pass into the liver, where a portion of the amino acids are taken up and used; the remainder pass through into the systemic circulation and are utilized by the peripheral tissues. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 599, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
About 11 to 15 g of nitrogen are excreted each day in the urine of a healthy adult consuming 70 to 100 g of protein, mostly in the form of urea, with smaller contributions from ammonia, uric acid, creatinine, and some free amino acids. These are the end products of protein metabolism, with urea and ammonia arising from the partial oxidation of amino acids. Uric acid and creatinine are indirectly derived from amino acids as well. The removal of nitrogen from the individual amino acids and its conversion to a form that can be excreted by the kidney can be considered as a two-part process. The first step usually takes place by one of two types of enzymatic reactions: transamination or deamination. Transamination is a reversible reaction that uses ketoacid intermediates of glucose metabolism (e.g., pyruvate, oxaloacetate, and alpha-ketoglutarate) as recipients of the amino nitrogen. Most amino acids can take part in these reactions, with the result that their amino nitrogen is transferred to just three amino acids: alanine from pyruvate, aspartate from oxaloacetate, and glutamate from alpha-ketoglutarate. Unlike many amino acids, branched-chain amino acid transamination occurs throughout the body, particularly in skeletal muscle. Here the main recipients of amino nitrogen are alanine and glutamine (from pyruvate and glutamate, respectively), which then pass into the circulation. These serve as important carriers of nitrogen from the periphery (skeletal muscle) to the intestine and liver. In the small intestine, glutamine is extracted and metabolized to ammonia, alanine, and citrulline, which are then conveyed to the liver via the portal circulation. Nitrogen is also removed from amino acids by deamination reactions, which result in the formation of ammonia. A number of amino acids can be deaminated, either directly (histidine), by dehydration (serine, threonine), by way of the purine nucleotide cycle (aspartate), or by oxidative deamination (glutamate). ... Glutamate is also formed in the specific degradation pathways of arginine and lysine. Thus, nitrogen from any amino acid can be funneled into the two precursors of urea synthesis, ammonia and aspartate.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 603-604, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
Although it seems clear that the efficiency of dietary protein digestion (in the sense of removal of amino acids from the small intestinal lumen) is high, there is now good evidence to show that nutritionally significant quantities of indispensable amino acids are metabolized by the tissues of the splanchnic bed, including the mucosal cells of the intestine. Thus, less than 100% of the amino acids removed from the intestinal lumen appear in the peripheral circulation, and the quantities that are metabolized by the splanchnic bed vary among the amino acids, with intestinal threonine metabolism being particularly high.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 600, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
For more Absorption, Distribution and Excretion (Complete) data for L-Threonine (12 total), please visit the HSDB record page.
Hepatic
The evidence indicates that excess threonine is converted to carbohydrate, liver lipids, and carbon dioxide.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 730, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
L-Threonine is a large neutral amino acid that is indispensable. ... L-threonine does not take part in transamination reactions.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 730, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
Once the amino acid deamination products enter the tricarboxylic acid (TCA) cycle (also known as the citric acid cycle or Krebs cycle) or the glycolytic pathway, their carbon skeletons are also available for use in biosynthetic pathways, particularly for glucose and fat. Whether glucose or fat is formed from the carbon skeleton of an amino acid depends on its point of entry into these two pathways. If they enter as acetyl-CoA, then only fat or ketone bodies can be formed. The carbon skeletons of other amino acids can, however, enter the pathways in such a way that their carbons can be used for gluconeogenesis. This is the basis for the classical nutritional description of amino acids as either ketogenic or glucogenic (ie, able to give rise to either ketones [or fat] or glucose). Some amino acids produce both products upon degradation and so are considered both ketogenic and glucogenic.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 606, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
The threonine dehydrogenase (TDG) pathway is a significant route of threonine degradation, yielding glycine in experimental animals, but has not been accurately quantitated in humans. Therefore, the effect of a large excess of dietary threonine, given either as free amino acid (+Thr) or as a constituent of protein (+P-Thr), on threonine catabolism to CO(2) and to glycine was studied in six healthy adult males using a 4-h constant infusion of L-[1-(13)C]threonine and [(15)N]glycine. Gas chromatography-combustion isotope ratio mass spectrometry was used to determine [(13)C]glycine produced from labeled threonine. Threonine intakes were higher on +Thr and +P-Thr diets compared with control (126, 126, and 50 micromol x kg(-1) x h(-1), SD 8, P < 0.0001). Threonine oxidation to CO(2) increased threefold in subjects on +Thr and +P-Thr vs. control (49, 45, and 15 micromol x kg(-1) x h(-1), SD 6, P < 0.0001). Threonine conversion to glycine tended to be higher on +Thr and +P-Thr vs. control (3.5, 3.4, and 1.6 micromol x kg(-1) x h(-1), SD 1.3, P = 0.06). The TDG pathway accounted for only 7-11% of total threonine catabolism and therefore is a minor pathway in the human adult.
PMID:10780944 Darling PB et al; Am J Physiol Endocrinol Metab 278 (5): E877-84 (2000).
For more Metabolism/Metabolites (Complete) data for L-Threonine (8 total), please visit the HSDB record page.
L-Threonine is a precursor to the amino acids glycine and serine. It acts as a lipotropic in controlling fat build-up in the liver. May help combat mental illness and may be very useful in indigestion and intestinal malfunctions. Also, threonine prevents excessive liver fat. Nutrients are more readily absorbed when threonine is present.
Amino acids are selected for protein synthesis by binding with transfer RNA (tRNA) in the cell cytoplasm. The information on the amino acid sequence of each individual protein is contained in the sequence of nucleotides in the messenger RNA (mRNA) molecules, which are synthesized in the nucleus from regions of DNA by the process of transcription. The mRNA molecules then interact with various tRNA molecules attached to specific amino acids in the cytoplasm to synthesize the specific protein by linking together individual amino acids; this process, known as translation, is regulated by amino acids (e.g., leucine), and hormones. Which specific proteins are expressed in any particular cell and the relative rates at which the different cellular proteins are synthesized, are determined by the relative abundances of the different mRNAs and the availability of specific tRNA-amino acid combinations, and hence by the rate of transcription and the stability of the messages. From a nutritional and metabolic point of view, it is important to recognize that protein synthesis is a continuing process that takes place in most cells of the body. In a steady state, when neither net growth nor protein loss is occurring, protein synthesis is balanced by an equal amount of protein degradation. The major consequence of inadequate protein intakes, or diets low or lacking in specific indispensable amino acids relative to other amino acids (often termed limiting amino acids), is a shift in this balance so that rates of synthesis of some body proteins decrease while protein degradation continues, thus providing an endogenous source of those amino acids most in need. /Protein synthesis/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 601-602, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
The mechanism of intracellular protein degradation, by which protein is hydrolyzed to free amino acids, is more complex and is not as well characterized at the mechanistic level as that of synthesis. A wide variety of different enzymes that are capable of splitting peptide bonds are present in cells. However, the bulk of cellular proteolysis seems to be shared between two multienzyme systems: the lysosomal and proteasomal systems. The lysosome is a membrane-enclosed vesicle inside the cell that contains a variety of proteolytic enzymes and operates mostly at acid pH. Volumes of the cytoplasm are engulfed (autophagy) and are then subjected to the action of the protease enzymes at high concentration. This system is thought to be relatively unselective in most cases, although it can also degrade specific intracellular proteins. The system is highly regulated by hormones such as insulin and glucocorticoids, and by amino acids. The second system is the ATP-dependent ubiquitin-proteasome system, which is present in the cytoplasm. The first step is to join molecules of ubiquitin, a basic 76-amino acid peptide, to lysine residues in the target protein. Several enzymes are involved in this process, which selectively targets proteins for degradation by a second component, the proteasome. /Protein degradation/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 602, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
86
PharmaCompass offers a list of L-Threonine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right L-Threonine manufacturer or L-Threonine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred L-Threonine manufacturer or L-Threonine supplier.
PharmaCompass also assists you with knowing the L-Threonine API Price utilized in the formulation of products. L-Threonine API Price is not always fixed or binding as the L-Threonine Price is obtained through a variety of data sources. The L-Threonine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A L Threonine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of L Threonine, including repackagers and relabelers. The FDA regulates L Threonine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. L Threonine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of L Threonine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A L Threonine supplier is an individual or a company that provides L Threonine active pharmaceutical ingredient (API) or L Threonine finished formulations upon request. The L Threonine suppliers may include L Threonine API manufacturers, exporters, distributors and traders.
click here to find a list of L Threonine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A L Threonine DMF (Drug Master File) is a document detailing the whole manufacturing process of L Threonine active pharmaceutical ingredient (API) in detail. Different forms of L Threonine DMFs exist exist since differing nations have different regulations, such as L Threonine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A L Threonine DMF submitted to regulatory agencies in the US is known as a USDMF. L Threonine USDMF includes data on L Threonine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The L Threonine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of L Threonine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The L Threonine Drug Master File in Japan (L Threonine JDMF) empowers L Threonine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the L Threonine JDMF during the approval evaluation for pharmaceutical products. At the time of L Threonine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of L Threonine suppliers with JDMF on PharmaCompass.
A L Threonine CEP of the European Pharmacopoeia monograph is often referred to as a L Threonine Certificate of Suitability (COS). The purpose of a L Threonine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of L Threonine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of L Threonine to their clients by showing that a L Threonine CEP has been issued for it. The manufacturer submits a L Threonine CEP (COS) as part of the market authorization procedure, and it takes on the role of a L Threonine CEP holder for the record. Additionally, the data presented in the L Threonine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the L Threonine DMF.
A L Threonine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. L Threonine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of L Threonine suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing L Threonine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for L Threonine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture L Threonine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain L Threonine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a L Threonine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of L Threonine suppliers with NDC on PharmaCompass.
L Threonine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of L Threonine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right L Threonine GMP manufacturer or L Threonine GMP API supplier for your needs.
A L Threonine CoA (Certificate of Analysis) is a formal document that attests to L Threonine's compliance with L Threonine specifications and serves as a tool for batch-level quality control.
L Threonine CoA mostly includes findings from lab analyses of a specific batch. For each L Threonine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
L Threonine may be tested according to a variety of international standards, such as European Pharmacopoeia (L Threonine EP), L Threonine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (L Threonine USP).

