
Synopsis
Synopsis
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
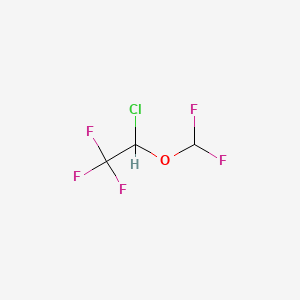

1. 26675-46-7
2. Forane
3. 1-chloro-2,2,2-trifluoroethyl Difluoromethyl Ether
4. 2-chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane
5. Forene
6. Aerrane
7. Isoflurano
8. Compound 469
9. Isoflo
10. Isofluranum
11. Ethane, 2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-
12. R-e 235dal
13. Terrell
14. Ether, 1-chloro-2,2,2-trifluoroethyl Difluoromethyl
15. Hsdb 8057
16. Cys9akd70p
17. Compound-469
18. Chebi:6015
19. 2-chloro-2-difluoromethoxy-1,1,1-trifluoroethane
20. Isofluranum [inn-latin]
21. Ncgc00181037-01
22. Aerrane (veterinary)
23. Dsstox_cid_752
24. Dsstox_rid_75769
25. Dsstox_gsid_20752
26. Isoba; Isofor; Isoforine; Isorrane; R-e 235da1
27. Isoflurano [inn-spanish]
28. Difluoromethyl 1-chloro-2,2,2-trifluoroethyl Ether (isoflurane)
29. Cas-26675-46-7
30. Forane (tn)
31. Ccris 3043
32. Isoflurane [anaesthetics, Volatile]
33. Einecs 247-897-7
34. Unii-cys9akd70p
35. Mfcd00066609
36. Brn 1852087
37. (+/-)-isoflurane
38. Isoflurane [usan:usp:inn:ban:jan]
39. Terrellhsdb 8057
40. Compd 469
41. Isoflurane, Aldrichcpr
42. Isoflurane [mi]
43. Isoflurane [inn]
44. Isoflurane [jan]
45. Isoflurane [usan]
46. Isoflurane [vandf]
47. Isoflurane [mart.]
48. Schembl1532
49. Chembl1256
50. Isoflurane [usp-rs]
51. Isoflurane [who-dd]
52. Difluoromethyl 1-chloro-2,2,2-trifluoroethyl Ether
53. Gtpl2505
54. Hsdb8057
55. Isoflurane (jp17/usp/inn)
56. Isoflurane [green Book]
57. Dtxsid3020752
58. Isoflurane [orange Book]
59. Hsdb-8057
60. Isoflurane [ep Monograph]
61. Bdbm217353
62. Isoflurane [usp Monograph]
63. Amy33546
64. Tox21_112685
65. Tox21_200831
66. Bbl100111
67. S6917
68. Stl454337
69. Akos006228574
70. Db00753
71. Ks-5166
72. Pb47772
73. Ncgc00181037-02
74. Ncgc00181037-03
75. Ncgc00258385-01
76. Ac-154802
77. Db-046999
78. Cs-0017450
79. Ft-0627416
80. C07518
81. D00545
82. P15338
83. A818554
84. Q413918
85. Sr-01000944965
86. Sr-01000944965-1
87. W-107162
88. 1-chloro-1-(difluoromethoxy)-2,2,2-trifluoroethane
89. 2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane
90. 1-chloro-2,2,2-trifluroethyl Difluromethyl Ether
91. Ethane, 1-chloro-1-(difluoromethoxy)-2,2,2-trifluoro-
92. 2-[bis(fluoranyl)methoxy]-2-chloranyl-1,1,1-tris(fluoranyl)ethane
| Molecular Weight | 184.49 g/mol |
|---|---|
| Molecular Formula | C3H2ClF5O |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 183.9714332 g/mol |
| Monoisotopic Mass | 183.9714332 g/mol |
| Topological Polar Surface Area | 9.2 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 102 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Forane |
| Drug Label | FORANE (isoflurane, USP), a nonflammable liquid administered by vaporizing, is a general inhalation anesthetic drug. It is 1-chloro-2, 2,2-trifluoroethyl difluoromethyl ether, and its structural formula is:Some physical constants are:Molecular weight... |
| Active Ingredient | Isoflurane |
| Dosage Form | Liquid |
| Route | Inhalation |
| Strength | 99.9% |
| Market Status | Prescription |
| Company | Baxter Hlthcare |
| 2 of 4 | |
|---|---|
| Drug Name | Isoflurane |
| Drug Label | Isoflurane, USP, is a nonflammable, nonexplosive general inhalation anesthetic agent. Its chemical name is 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether, and its structural formula is:Each mL contains 99.9% Isoflurane.Some physical const... |
| Active Ingredient | Isoflurane |
| Dosage Form | Liquid |
| Route | Inhalation |
| Strength | 99.9% |
| Market Status | Prescription |
| Company | Hospira; Halocarbon Prods; Piramal Critical; Piramal Ent |
| 3 of 4 | |
|---|---|
| Drug Name | Forane |
| Drug Label | FORANE (isoflurane, USP), a nonflammable liquid administered by vaporizing, is a general inhalation anesthetic drug. It is 1-chloro-2, 2,2-trifluoroethyl difluoromethyl ether, and its structural formula is:Some physical constants are:Molecular weight... |
| Active Ingredient | Isoflurane |
| Dosage Form | Liquid |
| Route | Inhalation |
| Strength | 99.9% |
| Market Status | Prescription |
| Company | Baxter Hlthcare |
| 4 of 4 | |
|---|---|
| Drug Name | Isoflurane |
| Drug Label | Isoflurane, USP, is a nonflammable, nonexplosive general inhalation anesthetic agent. Its chemical name is 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether, and its structural formula is:Each mL contains 99.9% Isoflurane.Some physical const... |
| Active Ingredient | Isoflurane |
| Dosage Form | Liquid |
| Route | Inhalation |
| Strength | 99.9% |
| Market Status | Prescription |
| Company | Hospira; Halocarbon Prods; Piramal Critical; Piramal Ent |
FORANE (isoflurane, USP) may be used for induction and maintenance of general anesthesia. Adequate data have not been developed to establish its application in obstetrical anesthesia.
US Natl Inst Health; DailyMed. Current Medication Information for Forane (isoflurane) inhalant (December 2011). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3d30eb8d-a62e-475f-926b-78ba63bee9c8
In cases of life-threatening status asthmaticus which are refractory to drug therapy, the administration of inhalation anesthetics can be life-saving as they help alleviate bronchial spasm. We had an 11-year-old female patient suffering from status asthmaticus who was moribund from severe CO2 narcosis and was not responding to any of the conventional therapies. She finally fell into ventricular fibrillation. After cardiopulmonary resuscitation, we administered 2.0% isoflurane in oxygen. Within half an hour, her high inspiratory pressure was dramatically decreased, and then the isoflurane concentration was maintained at 1.0%. After 14 hours of isoflurane anesthesia, PaCO2 decreased to the normal level and the isoflurane treatment was discontinued. The endotracheal tube was removed 4 hours later. She had an uneventful recovery and was discharged from the hospital 11 days later. With its low metabolic rate and therefore low organ toxicity, as well as its low arrhythmogenicity with remarkable bronchodilating activity, we feel isoflurane may well be superior to other inhalation anesthetics in the treatment of status asthmaticus.
PMID:8433481 Shibata Y et al; Masui 42 (1): 116-9 (1993)
Vet: Isoflurane, USP is used for induction and maintenance of general anesthesia in horses and dogs.
US Natl Inst Health; DailyMed. Current Medication Information for Isoflurane USP (isoflurane) inhalant (August 2009). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b0c8964f-24a8-4bab-ae8d-1536737e43ec
Anesthetics, Inhalation
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009) https://www.nlm.nih.gov/cgi/mesh/2012/MB_cgi?mode=&term=Isoflurane
In susceptible individuals, isoflurane anesthesia may trigger a skeletal muscle hypermetabolic state leading to high oxygen demand and the clinical syndrome known as malignant hyperthermia. The syndrome includes nonspecific features such as muscle rigidity, tachycardia, tachypnea, cyanosis, arrhythmias, and unstable blood pressure. (It should also be noted that many of these nonspecific signs may appear with light anesthesia, acute hypoxia, etc.) An increase in overall metabolism may be reflected in an elevated temperature, (which may rise rapidly early or late in the case, but usually is not the first sign of augmented metabolism) and an increased usage of the CO2 absorption system (hot canister). PaO2 and pH may decrease, and hyperkalemia and a base deficit may appear.
US Natl Inst Health; DailyMed. Current Medication Information for Forane (isoflurane) inhalant (December 2011). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3d30eb8d-a62e-475f-926b-78ba63bee9c8
Since levels of anesthesia may be altered easily and rapidly, only vaporizers producing predictable concentrations should be used. Hypotension and respiratory depression increase as anesthesia is deepened.
US Natl Inst Health; DailyMed. Current Medication Information for Forane (isoflurane) inhalant (December 2011). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3d30eb8d-a62e-475f-926b-78ba63bee9c8
Increased blood loss comparable to that seen with halothane has been observed in patients undergoing abortions.
US Natl Inst Health; DailyMed. Current Medication Information for Forane (isoflurane) inhalant (December 2011). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3d30eb8d-a62e-475f-926b-78ba63bee9c8
For more Drug Warnings (Complete) data for Isoflurane (24 total), please visit the HSDB record page.
For induction and maintenance of general anesthesia.
FDA Label
Sedation of mechanically ventilated patients
Isoflurane is a general inhalation anesthetic used for induction and maintenance of general anesthesia. It induces muscle relaxation and reduces pains sensitivity by altering tissue excitability. It does so by decreasing the extent of gap junction mediated cell-cell coupling and altering the activity of the channels that underlie the action potential.
Anesthetics, Inhalation
Gases or volatile liquids that vary in the rate at which they induce anesthesia; potency; the degree of circulation, respiratory, or neuromuscular depression they produce; and analgesic effects. Inhalation anesthetics have advantages over intravenous agents in that the depth of anesthesia can be changed rapidly by altering the inhaled concentration. Because of their rapid elimination, any postoperative respiratory depression is of relatively short duration. (From AMA Drug Evaluations Annual, 1994, p173) (See all compounds classified as Anesthetics, Inhalation.)
N - Nervous system
N01 - Anesthetics
N01A - Anesthetics, general
N01AB - Halogenated hydrocarbons
N01AB06 - Isoflurane
In the postanesthesia period, only 0.17% of the isoflurane taken up can be recovered as urinary metabolites.
US Natl Inst Health; DailyMed. Current Medication Information for Forane (isoflurane) inhalant (December 2011). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3d30eb8d-a62e-475f-926b-78ba63bee9c8
It is not known whether this drug is excreted in human milk.
US Natl Inst Health; DailyMed. Current Medication Information for Forane (isoflurane) inhalant (December 2011). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3d30eb8d-a62e-475f-926b-78ba63bee9c8
Minimal
Renal and hepatic toxicity of the fluorinated ether volatile anesthetics is caused by biotransformation to toxic metabolites. Metabolism also contributes significantly to the elimination pharmacokinetics of some volatile agents. Although innumerable studies have explored anesthetic metabolism in animals, there is little information on human volatile anesthetic metabolism with respect to comparative rates or the identity of the enzymes responsible for defluorination. The first purpose of this investigation was to compare the metabolism of the fluorinated ether anesthetics by human liver microsomes. The second purpose was to test the hypothesis that cytochrome P450 2E1 is the specific P450 isoform responsible for volatile anesthetic defluorination in humans. Microsomes were prepared from human livers. Anesthetic metabolism in microsomal incubations was measured by fluoride production. The strategy for evaluating the role of P450 2E1 in anesthetic defluorination involved three approaches: for a series of 12 human livers, correlation of microsomal defluorination rate with microsomal P450 2E1 content (measured by Western blot analysis), correlation of defluorination rate with microsomal P450 2E1 catalytic activity using marker substrates (para-nitrophenol hydroxylation and chlorzoxazone 6-hydroxylation), and chemical inhibition by P450 isoform-selective inhibitors. The rank order of anesthetic metabolism, assessed by fluoride production at saturating substrate concentrations, was methoxyflurane > sevoflurane > enflurane > isoflurane > desflurane > 0. There was a significant linear correlation of sevoflurane and methoxyflurane defluorination with antigenic P450 2E1 content (r = 0.98 and r = 0.72, respectively), but not with either P450 1A2 or P450 3A3/4. Comparison of anesthetic defluorination with either para-nitrophenol or chlorzoxazone hydroxylation showed a significant correlation for sevoflurane (r = 0.93, r = 0.95) and methoxyflurane (r = 0.78, r = 0.66). Sevoflurane defluorination was also highly correlated with that of enflurane (r = 0.93), which is known to be metabolized by human P450 2E1. Diethyldithiocarbamate, a selective inhibitor of P450 2E1, produced a concentration-dependent inhibition of sevoflurane, methoxyflurane, and isoflurane defluorination. No other isoform-selective inhibitor diminished the defluorination of sevoflurane, whereas methoxyflurane defluorination was inhibited by the selective P450 inhibitors furafylline (P450 1A2), sulfaphenazole (P450 2C9/10), and quinidine (P450 2D6) but to a much lesser extent than by diethyldithiocarbamate. These results demonstrate that cytochrome P450 2E1 is the principal, if not sole human liver microsomal enzyme catalyzing the defluorination of sevoflurane. P450 2E1 is the principal, but not exclusive enzyme responsible for the metabolism of methoxyflurane, which also appears to be catalyzed by P450s 1A2, 2C9/10, and 2D6. The data also suggest that P450 2E1 is responsible for a significant fraction of isoflurane metabolism. Identification of P450 2E1 as the major anesthetic metabolizing enzyme in humans provides a mechanistic understanding of clinical fluorinated ether anesthetic metabolism and toxicity.
PMID:8214760 Kharasch ED, Thummel KE; Anesthesiology 79 (4): 795-807 (1993)
Isoflurane undergoes minimal biotransformation in man.
US Natl Inst Health; DailyMed. Current Medication Information for Forane (isoflurane) inhalant (December 2011). Available from, as of July 23, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3d30eb8d-a62e-475f-926b-78ba63bee9c8
Renal and hepatic toxicity of the fluorinated ether volatile anesthetics is caused by biotransformation to toxic metabolites. Metabolism also contributes significantly to the elimination pharmacokinetics of some volatile agents. Although innumerable studies have explored anesthetic metabolism in animals, there is little information on human volatile anesthetic metabolism with respect to comparative rates or the identity of the enzymes responsible for defluorination. The first purpose of this investigation was to compare the metabolism of the fluorinated ether anesthetics by human liver microsomes. The second purpose was to test the hypothesis that cytochrome P450 2E1 is the specific P450 isoform responsible for volatile anesthetic defluorination in humans. Microsomes were prepared from human livers. Anesthetic metabolism in microsomal incubations was measured by fluoride production. The strategy for evaluating the role of P450 2E1 in anesthetic defluorination involved three approaches: for a series of 12 human livers, correlation of microsomal defluorination rate with microsomal P450 2E1 content (measured by Western blot analysis), correlation of defluorination rate with microsomal P450 2E1 catalytic activity using marker substrates (para-nitrophenol hydroxylation and chlorzoxazone 6-hydroxylation), and chemical inhibition by P450 isoform-selective inhibitors. The rank order of anesthetic metabolism, assessed by fluoride production at saturating substrate concentrations, was methoxyflurane > sevoflurane > enflurane > isoflurane > desflurane > 0. There was a significant linear correlation of sevoflurane and methoxyflurane defluorination with antigenic P450 2E1 content (r = 0.98 and r = 0.72, respectively), but not with either P450 1A2 or P450 3A3/4. Comparison of anesthetic defluorination with either para-nitrophenol or chlorzoxazone hydroxylation showed a significant correlation for sevoflurane (r = 0.93, r = 0.95) and methoxyflurane (r = 0.78, r = 0.66). Sevoflurane defluorination was also highly correlated with that of enflurane (r = 0.93), which is known to be metabolized by human P450 2E1. Diethyldithiocarbamate, a selective inhibitor of P450 2E1, produced a concentration-dependent inhibition of sevoflurane, methoxyflurane, and isoflurane defluorination. No other isoform-selective inhibitor diminished the defluorination of sevoflurane, whereas methoxyflurane defluorination was inhibited by the selective P450 inhibitors furafylline (P450 1A2), sulfaphenazole (P450 2C9/10), and quinidine (P450 2D6) but to a much lesser extent than by diethyldithiocarbamate. These results demonstrate that cytochrome P450 2E1 is the principal, if not sole human liver microsomal enzyme catalyzing the defluorination of sevoflurane. P450 2E1 is the principal, but not exclusive enzyme responsible for the metabolism of methoxyflurane, which also appears to be catalyzed by P450s 1A2, 2C9/10, and 2D6. The data also suggest that P450 2E1 is responsible for a significant fraction of isoflurane metabolism. Identification of P450 2E1 as the major anesthetic metabolizing enzyme in humans provides a mechanistic understanding of clinical fluorinated ether anesthetic metabolism and toxicity.
PMID:8214760 Kharasch ED, Thummel KE; Anesthesiology 79 (4): 795-807 (1993)
Isoflurane induces a reduction in junctional conductance by decreasing gap junction channel opening times and increasing gap junction channel closing times. Isoflurane also activates calcium dependent ATPase in the sarcoplasmic reticulum by increasing the fluidity of the lipid membrane. Also appears to bind the D subunit of ATP synthase and NADH dehydogenase. Isoflurane also binds to the GABA receptor, the large conductance Ca2+ activated potassium channel, the glutamate receptor and the glycine receptor.

Certificate Number : R1-CEP 2006-292 - Rev 00
Issue Date : 2012-09-24
Type : Chemical
Substance Number : 1673
Status : Valid
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18995
Submission : 2005-11-28
Status : Active
Type : II

Certificate Number : CEP 2010-078 - Rev 01
Issue Date : 2025-03-11
Type : Chemical
Substance Number : 1673
Status : Valid



GDUFA
DMF Review : Reviewed
Rev. Date : 2021-02-24
Pay. Date : 2021-01-11
DMF Number : 35361
Submission : 2020-12-22
Status : Active
Type : II



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9087
Submission : 1991-04-29
Status : Active
Type : II
Certificate Number : R1-CEP 2012-280 - Rev 00
Issue Date : 2019-04-11
Type : Chemical
Substance Number : 1673
Status : Withdrawn by Holder

USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 42149
Submission : 2025-06-22
Status : Active
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-01-30
Pay. Date : 2013-01-04
DMF Number : 9791
Submission : 1992-07-22
Status : Active
Type : II

USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 10363
Submission : 1993-07-19
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 12045
Submission : 1996-07-15
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
54
PharmaCompass offers a list of Isoflurane API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Isoflurane manufacturer or Isoflurane supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Isoflurane manufacturer or Isoflurane supplier.
PharmaCompass also assists you with knowing the Isoflurane API Price utilized in the formulation of products. Isoflurane API Price is not always fixed or binding as the Isoflurane Price is obtained through a variety of data sources. The Isoflurane Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Forene manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Forene, including repackagers and relabelers. The FDA regulates Forene manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Forene API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Forene manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Forene supplier is an individual or a company that provides Forene active pharmaceutical ingredient (API) or Forene finished formulations upon request. The Forene suppliers may include Forene API manufacturers, exporters, distributors and traders.
click here to find a list of Forene suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Forene DMF (Drug Master File) is a document detailing the whole manufacturing process of Forene active pharmaceutical ingredient (API) in detail. Different forms of Forene DMFs exist exist since differing nations have different regulations, such as Forene USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Forene DMF submitted to regulatory agencies in the US is known as a USDMF. Forene USDMF includes data on Forene's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Forene USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Forene suppliers with USDMF on PharmaCompass.
A Forene CEP of the European Pharmacopoeia monograph is often referred to as a Forene Certificate of Suitability (COS). The purpose of a Forene CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Forene EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Forene to their clients by showing that a Forene CEP has been issued for it. The manufacturer submits a Forene CEP (COS) as part of the market authorization procedure, and it takes on the role of a Forene CEP holder for the record. Additionally, the data presented in the Forene CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Forene DMF.
A Forene CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Forene CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Forene suppliers with CEP (COS) on PharmaCompass.
A Forene written confirmation (Forene WC) is an official document issued by a regulatory agency to a Forene manufacturer, verifying that the manufacturing facility of a Forene active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Forene APIs or Forene finished pharmaceutical products to another nation, regulatory agencies frequently require a Forene WC (written confirmation) as part of the regulatory process.
click here to find a list of Forene suppliers with Written Confirmation (WC) on PharmaCompass.
Forene Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Forene GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Forene GMP manufacturer or Forene GMP API supplier for your needs.
A Forene CoA (Certificate of Analysis) is a formal document that attests to Forene's compliance with Forene specifications and serves as a tool for batch-level quality control.
Forene CoA mostly includes findings from lab analyses of a specific batch. For each Forene CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Forene may be tested according to a variety of international standards, such as European Pharmacopoeia (Forene EP), Forene JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Forene USP).

