
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
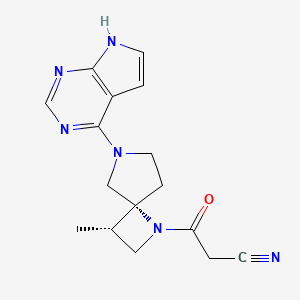

1. 1,6-diazaspiro(3.4)octane-1-propanenitrile, 3-methyl-beta-oxo-6-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-, (3s,4r)-
2. 3-((3s,4r)-3-methyl-6-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-1,6-diazaspiro(3.4)octan-1-yl)-3-oxopropanenitrile
3. Jte-052
1. 1263774-59-9
2. Jte-052
3. Jte-052a
4. Corectim
5. Leo 124249a
6. Leo 124249
7. Leo-124249a
8. Leo-124249
9. Delgocitinib [usan]
10. 9l0q8kk220
11. 3-[(3s,4r)-3-methyl-7-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1,7-diazaspiro[3.4]octan-1-yl]-3-oxopropanenitrile
12. 3-((3s,4r)-3-methyl-6-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-1,6-diazaspiro(3.4)octan-1-yl)-3-oxopropanenitrile
13. 3-[(3s,4r)-3-methyl-7-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1,7-diazaspiro[3.4]octan-1-yl]-3-oxidanylidene-propanenitrile
14. Delgocitinibum
15. Corectim (tn)
16. 3-((3s,4r)-3-methyl-6-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1,6-diazaspiro[3.4]octan-1-yl)-3-oxopropanenitrile
17. 3-[(3s,4r)-3-methyl-6-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1,6-diazaspiro[3.4]octan-1-yl]-3-oxopropanenitrile
18. Ent-60
19. Delgocitinib (jan/usan)
20. Delgocitinib [inn]
21. Delgocitinib [jan]
22. Delgocitinib [who-dd]
23. Unii-9l0q8kk220
24. Gtpl9619
25. Jte052
26. Chembl4297507
27. Schembl12547007
28. Chebi:167600
29. Dtxsid401336933
30. Ex-a5577
31. Bdbm50545650
32. At24880
33. Leo-124249;jte-052
34. Bd178539
35. Hy-109053
36. Cs-0031558
37. D11046
38. A936874
39. 1,6-diazaspiro(3.4)octane-1-propanenitrile, 3-methyl-.beta.-oxo-6-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-, (3s,4r)-
40. 1,6-diazaspiro(3.4)octane-1-propanenitrile, 3-methyl-beta-oxo-6-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-, (3s,4r)-
41. Fhx
| Molecular Weight | 310.35 g/mol |
|---|---|
| Molecular Formula | C16H18N6O |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 310.15420922 g/mol |
| Monoisotopic Mass | 310.15420922 g/mol |
| Topological Polar Surface Area | 88.9 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 544 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of dermatitis and eczema
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.




 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
About the Company : Biophore, founded in 2007, develops and manufactures niche and complex pharmaceutical products. With USFDA- and EU-approved API facilities, a dedicated intermediates site and an R&...
About the Company : Maithri Drugs Pvt. Ltd. is a global supplier of Active Pharmaceutical Ingredients (APIs), serving pharmaceutical companies in 60+ countries. Its API portfolio spans antivirals, ant...
About the Company : ApiSyn Healthcare, established in 2017, is a vertically integrated manufacturer of APIs & intermediates serving the global pharmaceutical industry. As part of SynZeal Research, a p...
About the Company : Conscientia Industrial is a pharmaceutical company, mainly developing, manufacturing, marketing APIs (Active Pharmaceutical Ingredients), intermediates, fine chemicals in China. We...

About the Company : Founded by chemists with years of working experiences in famous CRO companies in China, Excenen Pharmatech experienced in organic chemistry expecially in medicinal chemistry resear...

About the Company : FandaChem, headquartered in Hangzhou, China, is a reputable supplier and exporter of chemicals with a focus on delivering high-quality products. Our expertise lies in exporting a w...

About the Company : Sun-shine chemical has over 1,500m² of operation premises. The R&D laboratory is more than 1,000m² and is equipped with a variety of R&D laboratory equipment. The quality control...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Delgocitinib is a small molecule drug, which is currently being evaluated in Phase III clinical studies for the treatment of lichen sclerosus et atrophicus.
Lead Product(s): Delgocitinib,Inapplicable
Therapeutic Area: Dermatology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 13, 2026

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delgocitinib,Inapplicable
Therapeutic Area : Dermatology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
A Trial to Learn if Delgocitinib Can Help Reduce Symptoms in People With Lichen Sclerosus
Details : Delgocitinib is a small molecule drug, which is currently being evaluated in Phase III clinical studies for the treatment of lichen sclerosus et atrophicus.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
January 13, 2026

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Delgocitinib, a Janus Kinase (JAK) inhibitor, shows promise in treating Chronic Hand Eczema.
Lead Product(s): Delgocitinib,Inapplicable
Therapeutic Area: Dermatology Brand Name: Anzupgo
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 15, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delgocitinib,Inapplicable
Therapeutic Area : Dermatology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Leo Pharma Seeks Adolescent Label Expansion for Anzupgo® from EMA
Details : Delgocitinib, a Janus Kinase (JAK) inhibitor, shows promise in treating Chronic Hand Eczema.
Product Name : Anzupgo
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 15, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Delgocitinib is a Other Small Molecule drug candidate, which is currently being evaluated in Phase II clinical studies for the treatment of Psoriasis.
Lead Product(s): Delgocitinib,Inapplicable
Therapeutic Area: Dermatology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 10, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delgocitinib,Inapplicable
Therapeutic Area : Dermatology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Delgocitinib Cream 20 mg/g Safety and Efficacy In Palmoplantar Pustulosis
Details : Delgocitinib is a Other Small Molecule drug candidate, which is currently being evaluated in Phase II clinical studies for the treatment of Psoriasis.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 10, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Anzupgo (delgocitinib) cream is an investigational, first-in-class, topical pan-JAK inhibitor for CHE. It is under clinical development for the treatment of Moderate to Severe Chronic Hand Eczema.
Lead Product(s): Delgocitinib,Inapplicable
Therapeutic Area: Dermatology Brand Name: Anzupgo
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 27, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delgocitinib,Inapplicable
Therapeutic Area : Dermatology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
LEO Pharma’s Anzupgo Meets Endpoint in DELTA China Trial
Details : Anzupgo (delgocitinib) cream is an investigational, first-in-class, topical pan-JAK inhibitor for CHE. It is under clinical development for the treatment of Moderate to Severe Chronic Hand Eczema.
Product Name : Anzupgo
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 27, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Anzupgo (delgocitinib) cream is an investigational, first-in-class, topical pan-JAK inhibitor for CHE. It is under clinical development for the treatment of Moderate to Severe Chronic Hand Eczema.
Lead Product(s): Delgocitinib,Inapplicable
Therapeutic Area: Dermatology Brand Name: Anzupgo
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 04, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delgocitinib,Inapplicable
Therapeutic Area : Dermatology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
LEO’s Anzupgo® Achieves Positive DELTA TEEN Trial in Chronic Hand Eczema
Details : Anzupgo (delgocitinib) cream is an investigational, first-in-class, topical pan-JAK inhibitor for CHE. It is under clinical development for the treatment of Moderate to Severe Chronic Hand Eczema.
Product Name : Anzupgo
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 04, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Anzupgo (delgocitinib) cream is an investigational, first-in-class, topical pan-JAK inhibitor. It is indicated for the treatment of Moderate to Severe Chronic Hand Eczema.
Lead Product(s): Delgocitinib,Inapplicable
Therapeutic Area: Dermatology Brand Name: Undisclosed
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 14, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delgocitinib,Inapplicable
Therapeutic Area : Dermatology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
LEO Pharma’s Anzupgo® (delgocitinib) Cream Achieves Marketing Approval in Switzerland
Details : Anzupgo (delgocitinib) cream is an investigational, first-in-class, topical pan-JAK inhibitor. It is indicated for the treatment of Moderate to Severe Chronic Hand Eczema.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
November 14, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Anzupgo (delgocitinib) cream is an investigational, first-in-class, topical pan-JAK inhibitor. It is indicated for the treatment of Moderate to Severe Chronic Hand Eczema.
Lead Product(s): Delgocitinib,Inapplicable
Therapeutic Area: Dermatology Brand Name: Undisclosed
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delgocitinib,Inapplicable
Therapeutic Area : Dermatology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Germany Becomes The First Country to Launch LEO Pharma’s Anzupgo® (delgocitinib) Cream
Details : Anzupgo (delgocitinib) cream is an investigational, first-in-class, topical pan-JAK inhibitor. It is indicated for the treatment of Moderate to Severe Chronic Hand Eczema.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
October 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Anzupgo (delgocitinib) cream is a topical pan-JAK inhibitor. It is indicated for the treatment of adult patients with moderate to severe chronic hand eczema.
Lead Product(s): Delgocitinib,Inapplicable
Therapeutic Area: Dermatology Brand Name: Undisclosed
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delgocitinib,Inapplicable
Therapeutic Area : Dermatology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
EU Approves LEO Pharma’s Anzupgo® For Chronic Hand Eczema in Adults
Details : Anzupgo (delgocitinib) cream is a topical pan-JAK inhibitor. It is indicated for the treatment of adult patients with moderate to severe chronic hand eczema.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
September 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Anzupgo (delgocitinib) cream is an investigational, first-in-class, topical pan-JAK inhibitor for CHE. It is under clinical development for the treatment of Moderate to Severe Chronic Hand Eczema.
Lead Product(s): Delgocitinib,Inapplicable
Therapeutic Area: Dermatology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delgocitinib,Inapplicable
Therapeutic Area : Dermatology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
FDA Accepts LEO Pharma’s NDA for Delgocitinib Cream for Chronic Hand Eczema
Details : Anzupgo (delgocitinib) cream is an investigational, first-in-class, topical pan-JAK inhibitor for CHE. It is under clinical development for the treatment of Moderate to Severe Chronic Hand Eczema.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
September 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Anzupgo (delgocitinib) cream is an investigational, first-in-class, topical pan-JAK inhibitor for CHE. It is under clinical development for the treatment of Moderate to Severe Chronic Hand Eczema.
Lead Product(s): Delgocitinib,Inapplicable
Therapeutic Area: Dermatology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 25, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Delgocitinib,Inapplicable
Therapeutic Area : Dermatology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
LEO Pharma Receives Positive CHMP Opinion for Anzupgo® in Hand Eczema Treatment
Details : Anzupgo (delgocitinib) cream is an investigational, first-in-class, topical pan-JAK inhibitor for CHE. It is under clinical development for the treatment of Moderate to Severe Chronic Hand Eczema.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
July 25, 2024

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]CAS Number : 2491-20-5
End Use API : Delgocitinib
About The Company : Blue Jet Healthcare is a global, science-driven pharmaceutical company specializing in collaboration, development, and manufacturing of advanced pharmaceutical ...

1-Cyanoacetyl-3,5-dimethylpyrazole
CAS Number : 36140-83-7
End Use API : Delgocitinib
About The Company : Sunway Pharm ltd provides high quality building blocks to the global pharmaceutical and bio-technology industry. Combining our chemistry expertise and our econo...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
28
PharmaCompass offers a list of Delgocitinib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Delgocitinib manufacturer or Delgocitinib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Delgocitinib manufacturer or Delgocitinib supplier.
PharmaCompass also assists you with knowing the Delgocitinib API Price utilized in the formulation of products. Delgocitinib API Price is not always fixed or binding as the Delgocitinib Price is obtained through a variety of data sources. The Delgocitinib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Delgocitinib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Delgocitinib, including repackagers and relabelers. The FDA regulates Delgocitinib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Delgocitinib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Delgocitinib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Delgocitinib supplier is an individual or a company that provides Delgocitinib active pharmaceutical ingredient (API) or Delgocitinib finished formulations upon request. The Delgocitinib suppliers may include Delgocitinib API manufacturers, exporters, distributors and traders.
click here to find a list of Delgocitinib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Delgocitinib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Delgocitinib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Delgocitinib GMP manufacturer or Delgocitinib GMP API supplier for your needs.
A Delgocitinib CoA (Certificate of Analysis) is a formal document that attests to Delgocitinib's compliance with Delgocitinib specifications and serves as a tool for batch-level quality control.
Delgocitinib CoA mostly includes findings from lab analyses of a specific batch. For each Delgocitinib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Delgocitinib may be tested according to a variety of international standards, such as European Pharmacopoeia (Delgocitinib EP), Delgocitinib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Delgocitinib USP).

