
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Australia
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
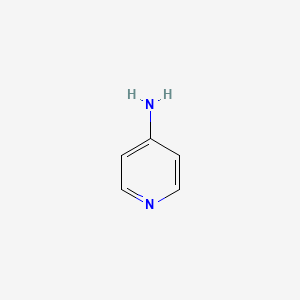

1. 4 Aminopyridine
2. 4 Aminopyridine Sustained Release
3. 4-aminopyridine Sustained Release
4. Dalfampridine
5. Fampridine Sr
6. Fampridine-sr
7. Pymadine
8. Sustained Release, 4-aminopyridine
9. Vmi 103
10. Vmi-103
11. Vmi103
1. 504-24-5
2. Pyridin-4-amine
3. Fampridine
4. 4-pyridinamine
5. Dalfampridine
6. 4-pyridylamine
7. P-aminopyridine
8. 4-ap
9. Avitrol
10. Ampyra
11. Gamma-aminopyridine
12. Avitrol 200
13. Pyridine, 4-amino-
14. Amino-4 Pyridine
15. Fampyra
16. Pimadin (free Base)
17. Compound 1861
18. Phillips 1861
19. Neurelan
20. Amaya
21. Prc 1237
22. Vmi 10-3
23. El-970
24. 4-amino-pyridine
25. 4- Aminopyridine
26. Pyridin-4-ylamine
27. Nsc 15041
28. Fampridine [inn]
29. .gamma.-aminopyridine
30. Dalfampridine [usan]
31. 4-aminopyrdine
32. N07xx07
33. Nsc-15041
34. Bh3b64okl9
35. Mls000069400
36. Chembl284348
37. Dalfampridine (4-aminopyridine)
38. Chebi:34385
39. 4-pyridinamine, Labeled With Deuterium
40. Dalfampridine (usan)
41. Ncgc00015009-08
42. Smr000058211
43. 4 Aminopyridine
44. Fampridine-sr
45. Dsstox_cid_3870
46. Dsstox_rid_77212
47. Dsstox_gsid_23870
48. Caswell No. 038
49. C5h6n2
50. Vmi-103
51. 4-amino Pyridine
52. Mi-w-3
53. 4ap
54. 916979-36-7
55. Cas-504-24-5
56. Ampyra (tn)
57. Hsdb 6037
58. Vmi 103
59. Sr-01000075299
60. Einecs 207-987-9
61. Rcra Waste No. P008
62. Unii-bh3b64okl9
63. Epa Pesticide Chemical Code 069201
64. Dalfampridine [usan:inn]
65. Brn 0105782
66. Fampridina
67. Fampridinum
68. Frampridine
69. Ampydin
70. Biib041
71. Pymadin
72. Ai3-52547
73. Biib 041
74. Fampridine-pr
75. Dalfampridine-er
76. 4-pyridyl Amine
77. Para-aminopyridine
78. Pyridine-4-amine
79. 3rxf
80. Neurelan (tn)
81. Pyridine-4-ylamine
82. Fampyra (tn)
83. Pyridin-4-yl-amine
84. Mfcd00006439
85. 4-aminopyridine 10
86. Philips 1861
87. Spectrum_000155
88. Tocris-0940
89. Fampridine (jan/inn)
90. 4-aminopyridine, 98%
91. Fampridine [jan]
92. Pyridine,4-amino
93. Opera_id_1768
94. Spectrum2_001413
95. Spectrum3_000914
96. Spectrum4_001013
97. Spectrum5_001501
98. Lopac-a-0152
99. Dalfampridine [ii]
100. Dalfampridine [mi]
101. Fampridine [vandf]
102. Upcmld-dp125
103. Wln: T6nj Dz
104. 4-aminopyridine (4-ap)
105. Fampridine [mart.]
106. 4-aminopyridine, >=99%
107. Dalfampridine [hsdb]
108. Fampridine [who-dd]
109. 1,4-dihydropyridin-4-imine
110. Lopac0_000032
111. Bspbio_001562
112. Dalfampridine [vandf]
113. Fampridine (4-aminopyridine)
114. Kbiogr_000282
115. Kbiogr_001505
116. Kbioss_000282
117. Kbioss_000635
118. 4-imino-1,4-dihydropyridine
119. 5-22-09-00106 (beilstein Handbook Reference)
120. Divk1c_000572
121. Fampridine [ema Epar]
122. Spectrum1501130
123. Spbio_001486
124. Dalfampridine [usp-rs]
125. 4 Ap
126. Biib-041
127. Gtpl2416
128. Cl-cob-iii-274-1
129. Dtxsid0023870
130. Upcmld-dp125:001
131. Ampd00207
132. Bdbm10458
133. Hms501m14
134. Kbio1_000572
135. Kbio2_000282
136. Kbio2_000635
137. Kbio2_002850
138. Kbio2_003203
139. Kbio2_005418
140. Kbio2_005771
141. Kbio3_000563
142. Kbio3_000564
143. Kbio3_001888
144. Ninds_000572
145. Bio1_000353
146. Bio1_000842
147. Bio1_001331
148. Bio2_000282
149. Bio2_000762
150. Hms1361o04
151. Hms1791o04
152. Hms1921h15
153. Hms1989o04
154. Hms2092f05
155. Hms2234n24
156. Hms3260g05
157. Hms3267e21
158. Hms3370j03
159. Hms3402o04
160. Hms3414h15
161. Hms3678h13
162. Hms3886i09
163. Kuc111877n
164. Pharmakon1600-01501130
165. Zinc599985
166. Dalfampridine [orange Book]
167. Act05167
168. Hy-b0604
169. Nsc15041
170. Vmi-10-3
171. Tox21_110065
172. Tox21_200793
173. Tox21_500032
174. Ccg-39031
175. Dalfampridine [usp Monograph]
176. Nsc757845
177. Rb8003
178. S5028
179. Stk298717
180. Akos000119896
181. Tox21_110065_1
182. Bs-3729
183. Db06637
184. Lp00032
185. Nsc-757845
186. Sdccgmls-0066228.p001
187. Sdccgsbi-0050021.p004
188. Idi1_000572
189. Idi1_034032
190. P-aminopyridine [un2671] [poison]
191. Ncgc00015009-01
192. Ncgc00015009-02
193. Ncgc00015009-03
194. Ncgc00015009-04
195. Ncgc00015009-05
196. Ncgc00015009-06
197. Ncgc00015009-07
198. Ncgc00015009-09
199. Ncgc00015009-10
200. Ncgc00015009-11
201. Ncgc00015009-12
202. Ncgc00015009-13
203. Ncgc00015009-14
204. Ncgc00015009-16
205. Ncgc00015009-23
206. Ncgc00015009-24
207. Ncgc00024890-01
208. Ncgc00024890-02
209. Ncgc00024890-03
210. Ncgc00024890-04
211. Ncgc00024890-05
212. Ncgc00024890-06
213. Ncgc00024890-07
214. Ncgc00024890-08
215. Ncgc00024890-09
216. Ncgc00024890-10
217. Ncgc00258347-01
218. Ncgc00260717-01
219. Bp-21343
220. Sbi-0050021.p003
221. Db-028705
222. A0414
223. Am20061261
224. B3530
225. Eu-0100032
226. Ft-0665455
227. Fampridine (4-aminopyridine) [vandf]
228. A 0152
229. D04127
230. Ab00052209_12
231. [j.pharmacol.exp.ther. 275:864 (1995)]
232. Ac-907/25014071
233. Q372539
234. 4-aminopyridine, Pestanal(r), Analytical Standard
235. Q-200436
236. Sr-01000075299-1
237. Sr-01000075299-3
238. Sr-01000075299-5
239. F2190-0487
240. Z955123498
241. Methyl3,5-dibromo-2,4-dihydroxy-6-methylbenzoate
242. Dalfampridine, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 94.11 g/mol |
|---|---|
| Molecular Formula | C5H6N2 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 94.053098200 g/mol |
| Monoisotopic Mass | 94.053098200 g/mol |
| Topological Polar Surface Area | 38.9 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 48 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Ampyra |
| PubMed Health | Dalfampridine (By mouth) |
| Drug Classes | Central Nervous System Agent |
| Active Ingredient | Dalfampridine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Acorda |
| 2 of 2 | |
|---|---|
| Drug Name | Ampyra |
| PubMed Health | Dalfampridine (By mouth) |
| Drug Classes | Central Nervous System Agent |
| Active Ingredient | Dalfampridine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Acorda |
Potassium Channel Blockers
National Library of Medicine's Medical Subject Headings. 4-Aminopyridine. Online file (MeSH, 2014). Available from, as of March 24, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Ampyra (dalfampridine) is a potassium channel blocker indicated to improve walking in patients with multiple sclerosis (MS). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for AMPYRA (dalfampridine) tablet, film coated, extended release (January 2014). Available from, as of March 25, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=550eb76a-e4a6-4fa1-ad65-c0fd8b0ce783
Distribution of dalfampridine is restricted; the drug is available only through certain specialty pharmacies.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3780
Anaphylactic reactions have been reported rarely in patients receiving dalfampridine. If an anaphylactic or other serious allergic reaction occurs, dalfampridine should be discontinued and should not be restarted.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3778
Dalfampridine can cause seizures. Postmarketing reports indicate that the majority of seizures have occurred in patients receiving the recommended dalfampridine dosage (generally within days to weeks after starting the drug) and in patients without a history of seizures. Some patients had been receiving other drugs that could have increased the risk of seizures or lowered the seizure threshold; in addition, age-related renal dysfunction and resultant increases in plasma dalfampridine concentrations could have contributed to the risk of seizures. Use of a high dalfampridine dosage (e.g., 15 or 20 mg twice daily) increases the risk of seizures. In open-label extension studies in patients with multiple sclerosis (MS), the incidence of seizures was more than 4 times greater at a dosage of 15 mg twice daily compared with that reported with the recommended dosage (10 mg twice daily).
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3778
Dalfampridine is contraindicated in patients with a prior history of seizures. The drug has not been evaluated in patients with a history of seizures or with evidence of epileptiform activity on EEG; such patients were excluded from clinical trials. The risk of seizures in patients with epileptiform activity on EEG is unknown and could be substantially higher than that observed in clinical trials.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3778
Urinary tract infections (UTIs) have been reported more frequently in patients receiving dalfampridine (12%) than in patients receiving placebo (8%). If a UTI occurs in a patient receiving dalfampridine, it should be evaluated and treated as clinically indicated.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3778
For more Drug Warnings (Complete) data for 4-AMINOPYRIDINE (12 total), please visit the HSDB record page.
Dalfampridine is a neurofunctional modifier that helps improve walking speed in patients with multiple sclerosis (MS).
FDA Label
Fampyra is indicated for the improvement of walking in adult patients with multiple sclerosis with walking disability (Expanded Disability Status Scale 4-7).
Multiple sclerosis with walking disability
Fampridine Accord is indicated for the improvement of walking in adult patients with multiple sclerosis with walking disability (EDSS 4-7).
Dalfampridine is a board-spectrum lipophillic potassium channel blocker and binds favourably to the open state than closed state of the potassium channel in the CNS. Its pharmacological target are the potassium channels exposed in MS patients. Does not prolong the QTc interval.
Potassium Channel Blockers
A class of drugs that act by inhibition of potassium efflux through cell membranes. Blockade of potassium channels prolongs the duration of ACTION POTENTIALS. They are used as ANTI-ARRHYTHMIA AGENTS and VASODILATOR AGENTS. (See all compounds classified as Potassium Channel Blockers.)
N07XX07
N07XX07
N07XX07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N07 - Other nervous system drugs
N07X - Other nervous system drugs
N07XX - Other nervous system drugs
N07XX07 - Fampridine
Absorption
Orally-administered dalfampridine is rapidly and completely absorbed from the gastrointestinal tract. Tmax, immediate release form = 1 hour; Tmax, extended release form = 3.5 hours; Cmax, 10 mg extended release = 17.3 - 21.6 ng/mL; Relative bioavailability of 10 mg extended-release tablets compared to aqueous oral solution = 96%
Route of Elimination
Almost all of the dose and its metabolites are completely eliminated by the kidneys after 24 hours. Urine (96%; 90% of total dose as unchanged drug); Feces (0.5%)
Volume of Distribution
10 mg extended release = 2.6 L/kg
Like other aminopyridines, 4-aminopyridine is rapidly absorbed from the gastrointestinal tract into circulation. The compound is readily metabolized in the liver and metabolites are excreted in urine. About 90% of the administered dose, following IV or oral administration, excretes in the urine.
Gupta, R. C. (ed.) Veterinary Toxicology: Basic and Clinical Principles. 1st ed. New York, NY, p.561-2 (2007)
Dalfampridine is rapidly and completely absorbed from the GI tract.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3779
The bioavailability of dalfampridine extended-release tablets is 96% compared with an extemporaneously prepared aqueous oral solution of immediate-release dalfampridine (formerly known as fampridine (4-aminopyridine, 4-AP)). Dalfampridine extended-release tablets result in delayed absorption and a slower increase to lower peak plasma concentrations compared with an aqueous oral solution of the drug, but the extent of absorption (area under the concentration-time curve (AUC)) is not affected. Plasma concentrations and AUC of dalfampridine increase proportionally with dose.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3779
The pharmacokinetics of dalfampridine in adults with multiple sclerosis (MS) are similar to that reported in healthy adults. In adults 29-56 years of age with MS who received a single 10-mg dalfampridine extended-release tablet, the mean peak plasma concentration was 25.23 ng/mL and was attained 3.92 hours after the dose. In healthy fasting adults, a single 10-mg extended-release tablet of the drug resulted in peak concentrations of 17.3-21.6 ng/mL and occurred 3-4 hours after the dose.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3779
For more Absorption, Distribution and Excretion (Complete) data for 4-AMINOPYRIDINE (8 total), please visit the HSDB record page.
Not extensively metabolized by the liver therefore drugs effecting the cytochrome P450 enzyme system that are concomitantly administered with dalfampridine are not expected to interact with each other. Metabolites include 3-hydroxy-4-aminopyridine and 3-hydroxy-4-aminopyridine sulfate and both are inactive. CYP2E1 is the enzyme responsible for 3-hydroxylation of dalfampridine.
The specific enzymes involved in the metabolism of fampridine were not identified in laboratory animals, but based on human microsome studies; it was suggested that CYP2E1 could be responsible for hydroxylation in man. In rat, approximately 36% of the parent drug was removed by hepatic first-pass metabolism. Fampridine was metabolized primarily by hydroxylation, followed by sulfate conjugation. Two circulating metabolites were detected in mouse, rat, rabbit, dog and human plasma: 3-hydroxy-4-AP and 3-hydroxy-4-AP sulfate. Although these metabolites were identified in all species, more extensive metabolism was determined in rats and dogs than in humans. In mouse and rat plasma, it was demonstrated that 4-AP-N-oxide was also a circulating metabolite. In human plasma, two unidentified metabolites were present; however, these metabolites accounted for <2% of the radioactivity.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Fampyra (Fampridine), p.13 (2011). Available from, as of March 27, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002097/WC500109957.pdf
A small portion of dalfampridine dose is metabolized by cytochrome P-450 (CYP) isoenzymes to 3-hydroxy-4-aminopyridine and 3-hydroxy-4-aminopyridine sulfate. These metabolites have no pharmacologic activity on potassium channels. In vitro studies indicate CYP2E1 is the major enzyme responsible for 3-hydroxylation of dalfampridine; other unidentified CYP enzymes play a minor role in 3-hydroxylation of the drug.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3779
Immediate release form = 3.5 hours; Extended release form = 5.47 hours;
Elimination of fampridine was in a similar range between rats and dogs with a plasma half-life of 1-2 hr, but was slightly prolonged in humans.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Fampyra (Fampridine), p.13 (2011). Available from, as of March 27, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002097/WC500109957.pdf
The half-life of dalfampridine is 5.2-6.5 hours. The half-life of 3-hydroxy-4-aminopyridine sulfate is 7.6 hours. /in humans/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3779
In MS, axons are progressively demyelinated which exposes potassium channels. As a result, there is a leak of potassium ions which results in the repolarization of cells and a decrease in neuronal excitability. The overall impact is the impairment of neuromuscular transmission as it is harder to trigger an action potential. Dalfampridine inhibits voltage-gated potassium channels in the CNS to maintain the transmembrane potential and prolong action potential. In other words, dalfampridine works to make sure that the current available is high enough to stimulate conduction in demyelinated axons that are exposed in MS patients. Furthermore, it facilitates neuromuscular and synaptic transmission by relieving conduction blocks in demyelinated axons.
Electrophysiological dysfunction of Purkinje cells causes cerebellar ataxia. Recent studies indicated that 4-aminopyridine (4-AP) can prevent the attacks in patients with episodic ataxia type 2. However, the cellular mechanism(s) by which 4-AP might be beneficial for the improvement of motor function remain unclear. Here, electrophysiological and behavioral consequences of in vivo co-treatment with 4-AP against 3-acetylpyridine (3-AP)-induced ataxia in rats were assessed. Combined treatment with 4-AP partially improved motor behavior compared to the ataxic rats. Treatment with 3-AP alone induced plastic alterations in the cells' intrinsic properties, so that the latency of the initial neural spike was significantly increased (Pb 0.001); however, both instantaneous firing frequency and amplitude of calcium spikes were significantly (Pb 0.001) suppressed. 3-AP treatment also resulted in significant decrease in the duration of action potential (Pb 0.05) and the amplitude of after hyperpolarization (Pb 0.05) as well as post-stimulus hyperpolarization potentials (Pb 0.001). Purkinje cells in rats co-treated with 4-AP, however, fired predominantly in rhythmic bursts. The mean amplitude of Ca2+ spikes was significantly (Pb 0.001) greater compared to ataxic rats, but similar to control value. As evidenced by a significant decrease (Pb 0.001) in the first spike latency, the cells' intrinsic excitability was also increased. In 4-AP co-treated group, the duration of action potential was also significantly lengthened (Pb 0.001) compared to control and 3-AP group. These results suggest that modulation of intrinsic electrical properties and potentiation of Ca2+ channels function caused by in vivo 4-AP treatment is likely to be partly responsible for its neuroprotective action.
PMID:20638947 Goudarzi I et al; Eur J Pharmacol 642 (1-3): 56-65 (2010)
4-Aminopyridine enhances the release of acetylcholine pre synaptically, increasing the force of muscle contraction. It antagonizes the neuromuscular blockade produced by many antibiotics. 4-Aminopyridine is relatively free of muscarinic side effects." 4-Aminopyridine readily crosses the blood brain barrier. It may be useful as an antagonist of nondepolarizing neuromuscular blocking agents such as d-tubocurarine, gallamine, pancuronium, atracurium, vecuronium, doxacurium, and pipecuronium.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 957
4-Aminopyridine blocks potassium ion channels and increases acetylcholine (ACh) levels at the synapses and neuromuscular junctions.
Gupta, R. C. (ed.) Veterinary Toxicology: Basic and Clinical Principles. 1st ed. New York, NY, p.562 (2007)
4-Aminopyridine block voltage-dependent potassium channels, producing secondary effects on calcium channels and influx of the cation, and this promotes release of acetylcholine.
Harvey, A.L. (ed.). Natural and Synthetic Neurotoxins. London, England: Academic Press 1993., p. 301
For more Mechanism of Action (Complete) data for 4-AMINOPYRIDINE (11 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : DALFAMPRIDINE
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG
Approval Date : 2018-07-11
Application Number : 206863
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : DALFAMPRIDINE
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG
Approval Date : 2017-01-23
Application Number : 206836
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

Brand Name : DALFAMPRIDINE
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG
Approval Date : 2018-07-30
Application Number : 206765
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : DALFAMPRIDINE
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG
Approval Date : 2017-01-23
Application Number : 206811
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : DALFAMPRIDINE
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG
Approval Date : 2018-10-24
Application Number : 206646
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
Brand Name : AMPYRA
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG
Approval Date : 2010-01-22
Application Number : 22250
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : DALFAMPRIDINE
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG
Approval Date : 2019-03-11
Application Number : 210158
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : DALFAMPRIDINE
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG
Approval Date : 2020-07-06
Application Number : 206858
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : DALFAMPRIDINE
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG
Approval Date : 2019-05-21
Application Number : 208292
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : DALFAMPRIDINE
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG
Approval Date :
Application Number : 206854
RX/OTC/DISCN :
RLD :
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Reply
13 Feb 2020
Reply
31 Jan 2020
Reply
01 Jan 2020
Reply
11 Dec 2019
Reply
16 Feb 2019
Reply
14 Aug 2018

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
Patent Expiration Date : 2025-04-11
Date Granted : 2015-01-27
Brand Name : FAMPYRA
Patent Number : 2562277
Filing Date : 2005-04-11
Strength per Unit : 10 mg
Dosage Form : TABLET (EXTENDED-RELEASE)
Human Or VET : Human
Route of Administration : ORAL
Patent Expiration Date : 2025-04-11
Date Granted : 2015-01-27

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

Dalfampridine Resolution Mixture (15 mg)
CAS Number :
Quantity Per Vial : 15
Sale Unit : mg
Price : $767.00
Details : Material Origin- Chemical Synthesis; USMCA- N...
Monograph :
Storage :
Code/Batch No : Catalog #1162443 / F00440

CAS Number : 504-24-5
Quantity Per Vial : 200
Sale Unit : mg
Price : $245.00
Details : Material Origin- Chemical Synthesis; USMCA- N...
Monograph :
Storage :
Code/Batch No : Catalog #1162454 / F0M557

Dalfampridine Related Compound A (25 mg) (4-A...
CAS Number : 3535-75-9
Quantity Per Vial : 25
Sale Unit : mg
Price : $777.00
Details : Material Origin- Chemical Synthesis; USMCA- N...
Monograph :
Storage :
Code/Batch No : Catalog #1162545 / F053K0

Dalfampridine Related Compound B (50 mg) (3,5...
CAS Number : 84539-34-4
Quantity Per Vial : 50
Sale Unit : mg
Price : $740.00
Details : Material Origin- Chemical Synthesis; USMCA- N...
Monograph :
Storage :
Code/Batch No : Catalog #1162556 / F053J0

Dalfampridine Related Compound C (25 mg) (1,3...
CAS Number : 39642-87-0
Quantity Per Vial : 25
Sale Unit : mg
Price : $740.00
Details : Material Origin- Chemical Synthesis; USMCA- N...
Monograph :
Storage :
Code/Batch No : Catalog #1162567 / F053H0

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
10
PharmaCompass offers a list of Fampridine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fampridine manufacturer or Fampridine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fampridine manufacturer or Fampridine supplier.
PharmaCompass also assists you with knowing the Fampridine API Price utilized in the formulation of products. Fampridine API Price is not always fixed or binding as the Fampridine Price is obtained through a variety of data sources. The Fampridine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Dalfampridine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Dalfampridine, including repackagers and relabelers. The FDA regulates Dalfampridine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Dalfampridine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Dalfampridine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Dalfampridine supplier is an individual or a company that provides Dalfampridine active pharmaceutical ingredient (API) or Dalfampridine finished formulations upon request. The Dalfampridine suppliers may include Dalfampridine API manufacturers, exporters, distributors and traders.
click here to find a list of Dalfampridine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Dalfampridine DMF (Drug Master File) is a document detailing the whole manufacturing process of Dalfampridine active pharmaceutical ingredient (API) in detail. Different forms of Dalfampridine DMFs exist exist since differing nations have different regulations, such as Dalfampridine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Dalfampridine DMF submitted to regulatory agencies in the US is known as a USDMF. Dalfampridine USDMF includes data on Dalfampridine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Dalfampridine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Dalfampridine suppliers with USDMF on PharmaCompass.
A Dalfampridine written confirmation (Dalfampridine WC) is an official document issued by a regulatory agency to a Dalfampridine manufacturer, verifying that the manufacturing facility of a Dalfampridine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Dalfampridine APIs or Dalfampridine finished pharmaceutical products to another nation, regulatory agencies frequently require a Dalfampridine WC (written confirmation) as part of the regulatory process.
click here to find a list of Dalfampridine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Dalfampridine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Dalfampridine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Dalfampridine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Dalfampridine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Dalfampridine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Dalfampridine suppliers with NDC on PharmaCompass.
Dalfampridine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Dalfampridine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Dalfampridine GMP manufacturer or Dalfampridine GMP API supplier for your needs.
A Dalfampridine CoA (Certificate of Analysis) is a formal document that attests to Dalfampridine's compliance with Dalfampridine specifications and serves as a tool for batch-level quality control.
Dalfampridine CoA mostly includes findings from lab analyses of a specific batch. For each Dalfampridine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Dalfampridine may be tested according to a variety of international standards, such as European Pharmacopoeia (Dalfampridine EP), Dalfampridine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Dalfampridine USP).

