
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Canada
0
Australia
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
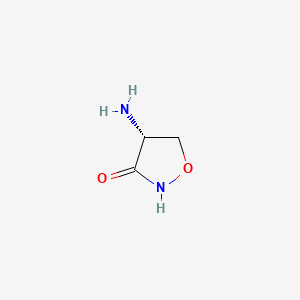

1. R-4-amino-3-isoxazolidinone
2. Seromycin
1. D-cycloserine
2. 68-41-7
3. Seromycin
4. Orientomycin
5. Oxamycin
6. Cyclo-d-serine
7. Cyclorin
8. D-4-amino-3-isoxazolidinone
9. Cicloserina
10. Farmiserina
11. Miroseryn
12. Tisomycin
13. Wasserina
14. Closina
15. Cycloserinum
16. (+)-4-amino-3-isoxazolidinone
17. D-4-amino-3-isoxazolidone
18. Alpha-cycloserine
19. (4r)-4-amino-1,2-oxazolidin-3-one
20. Miroserina
21. Tebemicina
22. Novoserin
23. (r)-4-aminoisoxazolidin-3-one
24. (+)-cycloserine
25. Oxamicina
26. D-(+)-cycloserine
27. (4r)-4-aminoisoxazolidin-3-one
28. Pa 94
29. Cycloserin
30. Micoserina
31. Pa-94
32. (r)-4-amino-isoxazolidin-3-one
33. D-oxamycin
34. Ro-1-9213
35. D-cs
36. 3-isoxazolidinone, 4-amino-, (r)-
37. E-733-a
38. 3-isoxazolidinone, 4-amino-, (4r)-
39. D-4-amino-3-isossazolidone
40. Hsdb 3218
41. D-oxamicina
42. 3-isoxazolidinone, 4-amino-, D-
43. K-300
44. I-1431
45. 3-isoxazolidinone, 4-amino-, (+)-
46. Nsc 154851
47. Chebi:40009
48. Ai3-50153
49. D-cycloserine, Synthetic
50. Dcs
51. Chembl771
52. Sc-49088
53. 95ik5ki84z
54. Nsc-76029
55. Nsc-154851
56. Cas-68-41-7
57. Ncgc00016306-01
58. Oxamicina [italian]
59. Cicloserina [italian]
60. Dsstox_cid_2870
61. Dsstox_rid_76766
62. Dsstox_gsid_22870
63. Cycloserinum [inn-latin]
64. Cicloserina [inn-spanish]
65. Cycloserine, D-
66. Closerin
67. .alpha.-cycloserine
68. Mfcd00005353
69. (r)-cycloserine
70. Seromycin (tn)
71. Smr000058313
72. D-4-amino-3-isossazolidone [italian]
73. (r)-4-amino-3-isoxazolidone
74. R-(+)-cycloserine
75. (r)-4-amino-3-isoxazolidinone
76. (4r)-4-amino-3-isoxazolidinone
77. Cycloserine (d)
78. Sr-01000075432
79. Drg-0195
80. (r)-(+)-cycloserine
81. Einecs 200-688-4
82. D-amino-3-isoxazolidinone
83. Brn 0080798
84. Unii-95ik5ki84z
85. Cycloserine-(d)
86. Serine, Cyclo-
87. 3-isoxazolidinone, 4-amino-, D
88. R(+)-4-amino-3-isoxazolidinone
89. Cycloserine [usp:inn:ban:jan]
90. 4ax
91. 3-isoxazolidinone, 4-amino-, (r)
92. (r)-(+)-4-amino-3-isoxazolidinone
93. Cycloserine, D(+)
94. D-cycloserine, Powder
95. Spectrum_000860
96. 1pb9
97. Cycloserine [mi]
98. Prestwick0_001089
99. Prestwick1_001089
100. Prestwick2_001089
101. Prestwick3_001089
102. Spectrum2_000084
103. Spectrum3_000371
104. Spectrum4_000305
105. Spectrum5_000797
106. Cycloserine [inn]
107. Cycloserine [jan]
108. Lopac-c-1159
109. Lopac-c-3909
110. Lopac-c-7005
111. Cycloserine [hsdb]
112. 3-isoxazolidinone, 4-amino-, (4r)- (9ci)
113. C 3909
114. C-9390
115. C-9400
116. Cycloserine [vandf]
117. Cycloserine [mart.]
118. Lopac0_000252
119. Schembl34322
120. Bspbio_001138
121. Bspbio_002121
122. Cycloserine [who-dd]
123. Cycloserine [who-ip]
124. Kbiogr_000890
125. Kbioss_001340
126. 4-27-00-05549 (beilstein Handbook Reference)
127. Mls000758215
128. Mls001423962
129. Mls002548887
130. Bidd:gt0707
131. D-cycloserine Synth. Bp 88
132. Divk1c_000098
133. Spectrum1500215
134. Spbio_000008
135. Spbio_003029
136. Bpbio1_001252
137. Fa6c7f8b-d080-4ea3-978f-1ecfb5a29d09
138. Gtpl9489
139. Cycloserine (jp17/usp/inn)
140. 4-amino-3-isoxazolidinone, D-
141. Dtxsid8022870
142. Hms500e20
143. Kbio1_000098
144. Kbio2_001340
145. Kbio2_003908
146. Kbio2_006476
147. Kbio3_001341
148. Cycloserine [orange Book]
149. Ninds_000098
150. Cycloserine [usp Impurity]
151. Hms1571i20
152. Hms1920c06
153. Hms2051c15
154. Hms2091i14
155. Hms2098i20
156. Hms2232f03
157. Hms3259l19
158. Hms3260d06
159. Hms3715i14
160. Nj-21
161. Pharmakon1600-01500215
162. Cycloserine [usp Monograph]
163. (r)-3-isoxazolidinone, 4-amino-
164. 4-amino-3-isoxazolidinone, (r)-
165. Act04767
166. Cycloserinum [who-ip Latin]
167. Hy-b0030
168. Tox21_110361
169. Tox21_500252
170. Bdbm50038178
171. Bdbm50103516
172. Ccg-39705
173. D-cycloserine, >=96.0% (nt)
174. Lmpk14000007
175. Nsc756712
176. S1998
177. Zinc34676245
178. 4-isoxazolidinamine, 3-oxo-, (d)-
179. Akos015994626
180. Tox21_110361_1
181. Ac-4721
182. Db00260
183. Hs-0079
184. Lp00252
185. Nc00050
186. Nc00676
187. Nsc-756712
188. Sdccgsbi-0050240.p005
189. Idi1_000098
190. Smp1_000167
191. Ncgc00015213-01
192. Ncgc00015213-02
193. Ncgc00015213-03
194. Ncgc00016306-02
195. Ncgc00016306-03
196. Ncgc00016306-04
197. Ncgc00016306-05
198. Ncgc00016306-07
199. Ncgc00016306-08
200. Ncgc00016306-17
201. Ncgc00093713-01
202. Ncgc00093713-02
203. Ncgc00260937-01
204. Cas-339-72-0
205. Sbi-0050240.p004
206. Ab00443920
207. Eu-0100252
208. 3-isoxazolidinone, 4-amino-, (+)- (8ci)
209. C08057
210. D00877
211. Ab00443920_09
212. Ab00443920_10
213. 005c353
214. A836140
215. Q418508
216. Sr-01000759389
217. Sr-01000075432-1
218. Sr-01000075432-2
219. Sr-01000075432-5
220. Sr-01000075432-9
221. Sr-01000759389-4
222. Sr-01000075432-10
223. F2173-1228
224. Z1522567171
225. Cycloserine, United States Pharmacopeia (usp) Reference Standard
226. Cycloserine, Pharmaceutical Secondary Standard; Certified Reference Material
227. (4r)-4-azaniumyl-4,5-dihydroisoxazol-3-olate;(r)-4-aminoisoxazolidin-3-one
| Molecular Weight | 102.09 g/mol |
|---|---|
| Molecular Formula | C3H6N2O2 |
| XLogP3 | -1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 102.042927438 g/mol |
| Monoisotopic Mass | 102.042927438 g/mol |
| Topological Polar Surface Area | 64.4 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 92.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Seromycin |
| PubMed Health | Cycloserine (By mouth) |
| Drug Classes | Antitubercular |
| Drug Label | Seromycin(Cycloserine Capsules, USP), 3-isoxazolidinone, 4-amino, (R)is a broadspectrum antibiotic that is produced by a strain of Streptomyces orchidaceus and has also been synthesized. Cycloserine is a white to offwhite powder that is soluble in wa... |
| Active Ingredient | Cycloserine |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 250mg |
| Market Status | Prescription |
| Company | Purdue Gmp |
| 2 of 2 | |
|---|---|
| Drug Name | Seromycin |
| PubMed Health | Cycloserine (By mouth) |
| Drug Classes | Antitubercular |
| Drug Label | Seromycin(Cycloserine Capsules, USP), 3-isoxazolidinone, 4-amino, (R)is a broadspectrum antibiotic that is produced by a strain of Streptomyces orchidaceus and has also been synthesized. Cycloserine is a white to offwhite powder that is soluble in wa... |
| Active Ingredient | Cycloserine |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 250mg |
| Market Status | Prescription |
| Company | Purdue Gmp |
Anti-Infective Agents, Urinary; Antibiotics, Antitubercular; Antimetabolites
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
INHIBITS WIDE VARIETY OF BOTH GRAM-POSITIVE & GRAM-NEGATIVE BACTERIA, INCL MYCOBACTERIA. ... IT HAS BEEN USED SUCCESSFULLY AGAINST STUBBORN URINARY TRACT INFECTIONS CAUSED BY STREPTOCOCCI, STAPHYLOCOCCI, E COLI & AEROBACTER AEROGENES.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1146
Cycloserine is indicated in combination with other antituberculars in the treatment of tuberculosis after failure of the primary medications (pyrazinamide, streptomycin, isoniazid, rifampin, and ethambutol). /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1130
Cycloserine is used in the treatment of atypical mycobacterial infections, such as mycobacterium avium complex. /NOT included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1130
For more Therapeutic Uses (Complete) data for CYCLOSERINE (6 total), please visit the HSDB record page.
PATIENTS WITH HISTORY OF MENTAL ILLNESS OFTEN TOLERATE CYCLOSERINE UNUSUALLY WELL, WHEREAS APPARENTLY STABLE INDIVIDUALS MAY DEVELOP PSYCHOTIC REACTION SOON AFTER INITIATION OF TREATMENT, SOMETIMES BEFORE THERAPEUTIC SERUM LEVELS ARE ACHIEVED.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 1641
Maternal Medication usually Compatible with Breast-Feeding: Cycloserine: Reported Sign or Symptom in Infant or Effect on Lactation: None. /from Table 6/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 140 (1994)
The drug may accumulate to toxic concentrations in patients with renal insufficiency; it may be removed from the circulation by dialysis.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1165
Because cycloserine is renally excreted, cycloserine may accumulate in patients with renal function impairment, leading to an increased risk of side effects; the medication should not be given to patients with renal function impairment (creatinine clearance of < 50 ml per minute (0.83 ml per second)).
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1131
For more Drug Warnings (Complete) data for CYCLOSERINE (10 total), please visit the HSDB record page.
Used in combination with up to 5 other drugs as a treatment for Mycobacterium avium complex (MAC) and is also used to treat tuberculosis (TB).
Cycloserine, a broad-spectrum antibiotic, may be bactericidal or bacteriostatic, depending on its concentration at the site of infection and the susceptibility of the organism. Cycloserine works by blocking the formation of these peptidoglycans. By doing this the walls of the bacteria become weak and it results in the death of the bacteria
Anti-Infective Agents, Urinary
Substances capable of killing agents causing urinary tract infections or of preventing them from spreading. (See all compounds classified as Anti-Infective Agents, Urinary.)
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
Antibiotics, Antitubercular
Substances obtained from various species of microorganisms that are, alone or in combination with other agents, of use in treating various forms of tuberculosis; most of these agents are merely bacteriostatic, induce resistance in the organisms, and may be toxic. (See all compounds classified as Antibiotics, Antitubercular.)
J - Antiinfectives for systemic use
J04 - Antimycobacterials
J04A - Drugs for treatment of tuberculosis
J04AB - Antibiotics
J04AB01 - Cycloserine
Absorption
Rapidly and almost completely absorbed (70 to 90%) from the gastrointestinal tract following oral administration.
VALUE OF CYCLOSERINE IS ENHANCED BY FACT THAT IT DIFFUSES INTO CELLS & CROSSES BLOOD-BRAIN BARRIER, EVEN IN ABSENCE OF DISEASE.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 1641
When given orally, 70% to 90% of cycloserine is rapidly absorbed. Peak concentrations in plasma are reached 3 to 4 hours after a single dose and are in the range of 20 to 35 ug/ml in children who receive 20 mg/kg; only small quantities are present after 12 hours.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1165
Cycloserine is distributed throughout body fluids and tissues. There is no appreciable blood-brain barrier to the drug, and CSF concentrations in all patients are approximately the same as those in plasma. About 50% of a parenteral dose of cycloserine is excreted unchanged in the urine in the first 12 hours; a total of 65% is recoverable in the active form over a period of 72 hours.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1165
APPROX 35% OF ANTIBIOTIC IS METABOLIZED TO AS-YET-UNIDENTIFIED SUBSTANCE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1211
Half-life in patients with normal renal function is 10 hours, and is prolonged in patients with impaired renal function.
Normal renal function - 10 hours. Impaired renal function - prolonged.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1131
Cycloserine is an analog of the amino acid D-alanine. It interferes with an early step in bacterial cell wall synthesis in the cytoplasm by competitive inhibition of two enzymes, L-alanine racemase, which forms D-alanine from L-alanine, and D-alanylalanine synthetase, which incorporates D-alanine into the pentapeptide necessary for peptidoglycan formation and bacterial cell wall synthesis.
EXCRETION OF B-ALANINE & D-BETA-AMINOISOBUTYRIC ACID WAS INCR IN PT WITH TUBERCULOSIS RECEIVING CLINICAL DOSES OF D-CYCLOSERINE.
YASUMITSU T ET AL; BIOCHEM PHARMACOL 25(3) 253-8 (1976)
Cycloserine is inhibitory for Mycobacterium tuberculosis in concentrations of 5 to 20 ug/ml in vitro. There is no cross-resistance between cycloserine and other tuberculostatic agents. While the antibiotic is effective in experimental infections caused by other microorganisms, studies in vitro reveal no suppression of growth in cultures made in conventional media, which contain D-alanine; this amino acid blocks the antibacterial activity of cycloserine. ... Cycloserine inhibits reactions in which D-alanine is involved in bacterial cell-wall synthesis. The use of media free of D-alanine reveals that the antibiotic inhibits the growth in vitro of enterococci, E. coli, Staph. aureus, Nocardia species, and Chlamydia.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1165

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
83
PharmaCompass offers a list of Cycloserine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Cycloserine manufacturer or Cycloserine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Cycloserine manufacturer or Cycloserine supplier.
PharmaCompass also assists you with knowing the Cycloserine API Price utilized in the formulation of products. Cycloserine API Price is not always fixed or binding as the Cycloserine Price is obtained through a variety of data sources. The Cycloserine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cycloserine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cycloserine, including repackagers and relabelers. The FDA regulates Cycloserine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cycloserine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Cycloserine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Cycloserine supplier is an individual or a company that provides Cycloserine active pharmaceutical ingredient (API) or Cycloserine finished formulations upon request. The Cycloserine suppliers may include Cycloserine API manufacturers, exporters, distributors and traders.
click here to find a list of Cycloserine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Cycloserine DMF (Drug Master File) is a document detailing the whole manufacturing process of Cycloserine active pharmaceutical ingredient (API) in detail. Different forms of Cycloserine DMFs exist exist since differing nations have different regulations, such as Cycloserine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Cycloserine DMF submitted to regulatory agencies in the US is known as a USDMF. Cycloserine USDMF includes data on Cycloserine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Cycloserine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Cycloserine suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Cycloserine Drug Master File in Korea (Cycloserine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Cycloserine. The MFDS reviews the Cycloserine KDMF as part of the drug registration process and uses the information provided in the Cycloserine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Cycloserine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Cycloserine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Cycloserine suppliers with KDMF on PharmaCompass.
A Cycloserine written confirmation (Cycloserine WC) is an official document issued by a regulatory agency to a Cycloserine manufacturer, verifying that the manufacturing facility of a Cycloserine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Cycloserine APIs or Cycloserine finished pharmaceutical products to another nation, regulatory agencies frequently require a Cycloserine WC (written confirmation) as part of the regulatory process.
click here to find a list of Cycloserine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Cycloserine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Cycloserine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Cycloserine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Cycloserine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Cycloserine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Cycloserine suppliers with NDC on PharmaCompass.
Cycloserine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cycloserine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cycloserine GMP manufacturer or Cycloserine GMP API supplier for your needs.
A Cycloserine CoA (Certificate of Analysis) is a formal document that attests to Cycloserine's compliance with Cycloserine specifications and serves as a tool for batch-level quality control.
Cycloserine CoA mostly includes findings from lab analyses of a specific batch. For each Cycloserine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cycloserine may be tested according to a variety of international standards, such as European Pharmacopoeia (Cycloserine EP), Cycloserine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cycloserine USP).

