
Synopsis
Synopsis
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
Australia
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
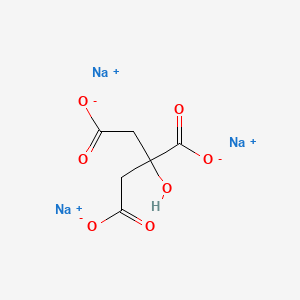

1. Anhydrous Sodium Citrate
2. Citra Ph
3. Monosodium Citrate
4. Sodium Citrate Dihydrate
5. Sodium Citrate Monobasic
6. Sodium Citrate, Anhydrous
7. Trisodium Citrate Dihydrate
1. Trisodium Citrate
2. 68-04-2
3. Citrosodine
4. Natrocitral
5. Sodium Citrate Anhydrous
6. Sodium Citrate, Anhydrous
7. Citric Acid, Trisodium Salt
8. Trisodium Citrate, Anhydrous
9. 1,2,3-propanetricarboxylic Acid, 2-hydroxy-, Trisodium Salt
10. Anhydrous Sodium Citrate
11. Citric Acid Trisodium Salt
12. Sodium 2-hydroxypropane-1,2,3-tricarboxylate
13. Trisodium Citrate Anhydrous
14. Fema No. 3026
15. Trisodium-citrate
16. Anhydrous Trisodium Citrate
17. 994-36-5
18. Sodium Citrate,anhydrous
19. Rs7a450lga
20. Ins No.331(iii)
21. Ins-331(iii)
22. Chebi:53258
23. E-331(iii)
24. Trisodium 2-hydroxypropane-1,2,3-tricarboxylate
25. Trisodium;2-hydroxypropane-1,2,3-tricarboxylate
26. Mfcd00012462
27. Fema No. 3026, Anhydrous-
28. Citric Acid, Sodium Salt
29. Citrosodina
30. Citnatin
31. Citreme
32. Citrosodna
33. Sodium Citrate Hydrous
34. 1,2,3-propanetricarboxylic Acid, 2-hydroxy-, Sodium Salt
35. Ccris 3293
36. Sodium Citrate (na3c6h5o7)
37. Hsdb 5201
38. Einecs 200-675-3
39. Unii-rs7a450lga
40. N-1560
41. Natrii Citras
42. Tri-sodium Citrate
43. Trisodium 2-hydroxy-1,2,3-propanetricarboxylate
44. Sodium Citrate Salt
45. 1,2,3-propanetricarboxylic Acid, 2-hydroxy-, Sodium Salt (1:3)
46. Sodium (iii) Citrate
47. Sodium Citrate (usp)
48. Natrii Citras, Dehydrate
49. Ec 200-675-3
50. Anticoagulant Sodium Citrate
51. Chembl1355
52. Sodium Citrate [mi]
53. Citrate Concentrated Solution
54. Dtxsid2026363
55. Sodium Citrate [who-ip]
56. 2-hydroxy-1,2,3-propanetricarboxylic Acid, Trisodium Salt
57. Citric Acid Trisodium Salt, 99%
58. Natrii Citras [who-ip Latin]
59. Akos015915009
60. Citrate Solution, Ph ~3.0, 30 Mm
61. Db09154
62. Sodium Citrate Anhydrous [hsdb]
63. Anhydrous Trisodium Citrate [ii]
64. Sodium Citrate,anhydrous [vandf]
65. Ac-15008
66. E331
67. Sodium Citrate Dihydrate Usp Fine Granular
68. Sodium Citrate, Anhydrous [who-ip]
69. B7298
70. Ft-0623960
71. D05855
72. D77308
73. Sodium Citrate, 0.5m Buffer Solution, Ph 5.0
74. Sodium Citrate, 0.5m Buffer Solution, Ph 5.5
75. Sodium Citrate, 0.5m Buffer Solution, Ph 6.0
76. Sodium Citrate, 0.5m Buffer Solution, Ph 6.5
77. Anhydrous Trisodium Citrate [usp Monograph]
78. Q409728
79. J-520101
80. Citric Acid Trisodium Salt, Anhydrous, >=98% (gc)
81. Citrate Solution, Ph 3.6+/-0.1 (25 C), 27 Mm
82. Citric Acid Trisodium Salt, Vetec(tm) Reagent Grade, 98%
83. 2-hydroxy-1,2,3-propanenetricarboxylic Acid Trisodium Salt Dihydrate
84. Citrate Concentrated Solution, Bioultra, For Molecular Biology, 1 M In H2o
85. Buffer Solution Ph 5.0 (20 C), Citric Acid ~0.096 M, Sodium Hydroxide ~0.20 M
86. Citrate Concentrated Solution, Bioreagent, Suitable For Coagulation Assays, 4 % (w/v)
87. 8055-55-8
| Molecular Weight | 258.07 g/mol |
|---|---|
| Molecular Formula | C6H5Na3O7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 257.97283534 g/mol |
| Monoisotopic Mass | 257.97283534 g/mol |
| Topological Polar Surface Area | 141 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 211 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
...USED AS AN EXPECTORANT...& SYSTEMIC ALKALIZER. SALINE EXPECTORANTS ARE ESPECIALLY USEFUL WHEN IT IS DESIRED TO LIQUEFY THICK, TENACIOUS SPUTUM. IN THE BODY, SODIUM CITRATE IS OXIDIZED TO BICARBONATE & EXCRETED IN THE URINE; THUS, WHEN GIVEN ORALLY IT IS USEFUL IN ACIDOSIS & TO OVERCOME EXCESSIVE URINARY ACIDITY.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 802
SODIUM CITRATE ALSO HAS A DIURETIC...ACTION.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 40:08
SODIUM CITRATE ALSO INCREASES THE URINARY EXCRETION OF CALCIUM. THEREFORE, IT HAS BEEN EMPLOYED IN HYPERCALCEMIA & TO FACILITATE ELIMINATION OF LEAD IN POISONING DUE TO THE LATTER AGENT. /SRP: FORMER USE/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 802
MEDICATION (VET): ANTICOAGULANT FOR COLLECTION OF BLOOD /SRP: FORMER USE/
The Merck Index. 9th ed. Rahway, New Jersey: Merck & Co., Inc., 1976., p. 1112
WHEN GIVEN IN EXCESS AMT SODIUM CITRATE MAY PRODUCE ALKALOSIS & MAY CAUSE TETANY OR DEPRESS THE HEART BY DECREASING THE IONIZED CALCIUM LEVEL OF THE BLOOD.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 40:08
Used as an anticoagulant during plasmophoresis as well as a neutralizing agent in the treatment of upset stomach and acidic urine.
FDA Label
Citrate prevents activation of the clotting cascade by chelating calcium ions. Citrate neutralizes acid in the stomach and urine, raising the pH.
Buffers
A chemical system that functions to control the levels of specific ions in solution. When the level of hydrogen ion in solution is controlled the system is called a pH buffer. (See all compounds classified as Buffers.)
Food Preservatives
Substances capable of inhibiting, retarding or arresting the process of fermentation, acidification or other deterioration of foods. (See all compounds classified as Food Preservatives.)
Anticoagulants
Agents that prevent BLOOD CLOTTING. (See all compounds classified as Anticoagulants.)
B - Blood and blood forming organs
B05 - Blood substitutes and perfusion solutions
B05C - Irrigating solutions
B05CB - Salt solutions
B05CB02 - Sodium citrate
Absorption
Tmax of 98-130min.
Route of Elimination
Largely eliminated through hepatic metabolism with very little cleared by the kidneys.
Volume of Distribution
19-39L.
Clearance
Total clearance of 313-1107mL/min.
IN THE BODY, SODIUM CITRATE IS OXIDIZED TO BICARBONATE & EXCRETED IN THE URINE...
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 802
Citrate is metabolized to bicarbonate in the liver and plays a role as an intermediate in the citric acid cycle.
IN THE BODY, SODIUM CITRATE IS OXIDIZED TO BICARBONATE...
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 802
18-54 min
Citrate chelates free calcium ions preventing them from forming a complex with tissue factor and coagulation factor VIIa to promote the activation of coagulation factor X. This inhibits the extrinsic initiation of the coagulation cascade. Citrate may also exert an anticoagulant effect via a so far unknown mechanism as restoration of calcium concentration does not fully reverse the effect of citrate. Citrate is a weak base and so reacts with hydrochloric acid in the stomach to raise the pH. It it further metabolized to bicarbonate which then acts as a systemic alkalizing agent, raising the pH of the blood and urine. It also acts as a diuretic and increases the urinary excretion of calcium.
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40880
Submission : 2024-11-22
Status : Active
Type : IV

Date of Issue : 2026-01-12
Valid Till : 2028-02-02
Written Confirmation Number : WC-0518
Address of the Firm :


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 41247
Submission : 2025-01-24
Status : Active
Type : IV


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 28694
Submission : 2014-09-29
Status : Active
Type : IV


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22551
Submission : 2009-02-19
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35752
Submission : 2021-03-29
Status : Active
Type : IV


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 31774
Submission : 2017-09-06
Status : Active
Type : IV


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 41123
Submission : 2025-06-05
Status : Active
Type : IV


GDUFA
DMF Review : Reviewed
Rev. Date : 2021-07-02
Pay. Date : 2021-06-02
DMF Number : 35887
Submission : 2021-05-11
Status : Active
Type : II


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
About the Company : Rochem, established in 1994, is a global distributor of pharmaceutical, food, nutritional, and animal health ingredients, sourcing high-quality products from China. Headquartered i...
About the Company : Jai Radhe Sales, founded in 1999, is a global distributor specializing in high-quality pharmaceutical ingredients from India. It offers complete sourcing solutions, technical and r...
Sodium citrate tribasic hydrate
About the Company : India Phosphate, established in 2007, is a leading processor, exporter, and supplier of high-quality Calcium Phosphate. Known for its purity, accuracy, and cost-effectiveness, our ...

About the Company : Incorporated in 1992, ISHITA DRUGS & INDUSTRIES LIMITED (IDIL) is the flagship company of ISHITA group. IDIL is a public limited company, listed on the domestic stock exchanges (BS...

About the Company : Jiaan Biotech is counted as a reliable Manufacturer and Supplier of quality oriented Mineral Supplements. The Manufacturing Unit is based in Pithampur Industrial Area , Madhya Prad...

About the Company : Nandu Group consisting of Nandu Chemical Industries, Nandu Chemicals Private Limited and Nandu Pharma Private Limited has a diversified product range to serve multiple applications...

About the Company : Progress is a company led by professionals, established by technocrats with a vision for global business expansion. Our headquarters are located in Vashi, Mumbai, with a manufactur...

About the Company : Sudeep Pharma Pvt. Ltd. is a leading producer of Calcium based Pharmaceutical, Food and Feed ingredients in India for the last quarter of a century. Established in 1989, we are one...

About the Company : Vasa Pharmachem Pvt. Ltd. established in the year 1988, is a part of the diversified Vasa Group of companies.Company was established to support a wide range of industries by manufa...

About the Company : VM Chemicals is one of the topmost Manufacturers, Exporters and Wholesale Suppliers of a wide gamut of Chemicals like Sodium Chloride IP BP, Sodium Bicarbonate IP BP, Sodium Acetat...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
Dosage Form : Cream / Lotion / Ointment
Grade : Topical, Parenteral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Application : Taste Masking
Excipient Details : Stevia is used as a sweetening agent in the production of oral dosage forms such as tablets.
Dosage Form : Solution
Grade : Oral
Application : Thickeners and Stabilizers
Excipient Details : Tapioca is used as a thickening agent and stabilizer in pharmaceutical liquid dosage form production.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : Microlose (Lactose Monohydrate & Microcrystalline Cellulose) is used as a diluent in oral dosage forms such as tablets.
Pharmacopoeia Ref : DMF, EXCiPAT, KOSHER, HALAL, W...
Technical Specs : Lactose Monohydrate – 40%, Microcrystalline cellulose – 60%
Ingredient(s) : Lactose Monohydrate
Dosage Form : Capsule
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : ProBlend (SMCC) is a co-processed excipient consists of microcrystalline cellulose & colloidal silicon dioxide, used as a diluent & binder in OSDs.
Pharmacopoeia Ref : USP-NF, DMF, EXCiPAT, KOSHER, ...
Technical Specs : NA
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Softgels
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Injectable / Parenteral
Grade : Parenteral
Application : Parenteral
Excipient Details : Castor oil is used as an oily solvent in parenteral dosage forms.
Dosage Form : Injectable / Parenteral
Grade : Parenteral, Oral, Topical
Dosage Form : Softgels
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Not Available
Brand Name : Kollicoat Protect
Application : Coating Systems & Additives
Excipient Details : Flexible water soluble instant release coating polymer, especially used for moisture protection
Pharmacopoeia Ref : Excipient based on Kollicoat®...
Technical Specs : Not Available
Ingredient(s) : Polyvinyl Alcohol Graft Polyethylene Glycol Copolymer
Dosage Form : Gel
Grade : Not Available
Brand Name : Kollisolv PEG 1000
Application : Topical
Excipient Details : Forms anhydrous, hydrophilic ointments in combination with low mol. weight PEG
Pharmacopoeia Ref : USP-NF
Technical Specs : Not Available
Ingredient(s) : Polyethylene Glycol Excipient
Dosage Form : Suppository
Grade : Not Available
Brand Name : Kollisolv PEG 1450
Application : Thickeners and Stabilizers
Excipient Details : Forms anhydrous, hydrophilic ointments in combination with low mol. weight PEG
Pharmacopoeia Ref : USP-NF
Technical Specs : Not Available
Ingredient(s) : Polyethylene Glycol Excipient
Dosage Form : Emulsion
Grade : Not Available
Brand Name : Kollisolv PEG 300
Application : Solubilizers
Excipient Details : Liquid plasticizer, solvent for oral and topical applications, hydrophilic fill for solubilization of hydrophilic APIs.
Pharmacopoeia Ref : Ph. Eur., JP, FCC, USP
Technical Specs : Not Available
Ingredient(s) : Polyethylene Glycol Excipient
Dosage Form : Suppository
Grade : Not Available
Brand Name : Kollisolv PEG 3350
Application : Thickeners and Stabilizers
Excipient Details : Forms anhydrous, hydrophilic ointments in combination with low mol. weight PEG
Pharmacopoeia Ref : USP-NF
Technical Specs : Not Available
Ingredient(s) : Polyethylene Glycol Excipient
Dosage Form : Tablet
Grade : Not Available
Brand Name : Kollisolv PEG 400
Application : Solubilizers
Excipient Details : Liquid plasticizer, Solvent for oral and topical applications, Hydrophilic fill for solubilization of hydrophilic APIs.
Pharmacopoeia Ref : Ph. Eur., JP, FCC, USP
Technical Specs : Not Available
Ingredient(s) : Polyethylene Glycol Excipient
Dosage Form : Softgel Capsule
Grade : Not Available
Brand Name : Kollisolv PEG 600
Application : Solubilizers
Excipient Details : Hydrophilic fill for solubilization of hydrophilic APIs.
Pharmacopoeia Ref : Ph. Eur., JP, FCC, USP
Technical Specs : Not Available
Ingredient(s) : Polyethylene Glycol Excipient
Dosage Form : Tablet
Grade : Not Available
Application : Film Formers & Plasticizers
Excipient Details : Liquid plasticizer with high ADI, hydrophilic solvent & humectant in emulsions, skin penetration enhancer in topical formulaitons.
Pharmacopoeia Ref : Ph. Eur., JP, FCC, USP
Technical Specs : Not Available
Ingredient(s) : Propylene Glycol
Dosage Form : Softgel Capsule
Grade : Not Available
Application : Thickeners and Stabilizers
Excipient Details : Structure-building consistency factor with dry feel, forms crystalline barrier on skin
Pharmacopoeia Ref : Ph. Eur., USP-NF, JP: Stearic ...
Technical Specs : Not Available
Ingredient(s) : Stearic Acid
Dosage Form : Tablet
Grade : Not Available
Application : Granulation
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
49
PharmaCompass offers a list of Sodium Citrate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sodium Citrate manufacturer or Sodium Citrate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sodium Citrate manufacturer or Sodium Citrate supplier.
PharmaCompass also assists you with knowing the Sodium Citrate API Price utilized in the formulation of products. Sodium Citrate API Price is not always fixed or binding as the Sodium Citrate Price is obtained through a variety of data sources. The Sodium Citrate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Citrosodina manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Citrosodina, including repackagers and relabelers. The FDA regulates Citrosodina manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Citrosodina API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Citrosodina manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Citrosodina supplier is an individual or a company that provides Citrosodina active pharmaceutical ingredient (API) or Citrosodina finished formulations upon request. The Citrosodina suppliers may include Citrosodina API manufacturers, exporters, distributors and traders.
click here to find a list of Citrosodina suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Citrosodina DMF (Drug Master File) is a document detailing the whole manufacturing process of Citrosodina active pharmaceutical ingredient (API) in detail. Different forms of Citrosodina DMFs exist exist since differing nations have different regulations, such as Citrosodina USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Citrosodina DMF submitted to regulatory agencies in the US is known as a USDMF. Citrosodina USDMF includes data on Citrosodina's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Citrosodina USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Citrosodina suppliers with USDMF on PharmaCompass.
A Citrosodina CEP of the European Pharmacopoeia monograph is often referred to as a Citrosodina Certificate of Suitability (COS). The purpose of a Citrosodina CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Citrosodina EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Citrosodina to their clients by showing that a Citrosodina CEP has been issued for it. The manufacturer submits a Citrosodina CEP (COS) as part of the market authorization procedure, and it takes on the role of a Citrosodina CEP holder for the record. Additionally, the data presented in the Citrosodina CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Citrosodina DMF.
A Citrosodina CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Citrosodina CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Citrosodina suppliers with CEP (COS) on PharmaCompass.
A Citrosodina written confirmation (Citrosodina WC) is an official document issued by a regulatory agency to a Citrosodina manufacturer, verifying that the manufacturing facility of a Citrosodina active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Citrosodina APIs or Citrosodina finished pharmaceutical products to another nation, regulatory agencies frequently require a Citrosodina WC (written confirmation) as part of the regulatory process.
click here to find a list of Citrosodina suppliers with Written Confirmation (WC) on PharmaCompass.
Citrosodina Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Citrosodina GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Citrosodina GMP manufacturer or Citrosodina GMP API supplier for your needs.
A Citrosodina CoA (Certificate of Analysis) is a formal document that attests to Citrosodina's compliance with Citrosodina specifications and serves as a tool for batch-level quality control.
Citrosodina CoA mostly includes findings from lab analyses of a specific batch. For each Citrosodina CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Citrosodina may be tested according to a variety of international standards, such as European Pharmacopoeia (Citrosodina EP), Citrosodina JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Citrosodina USP).

