
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
FDA Orange Book
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
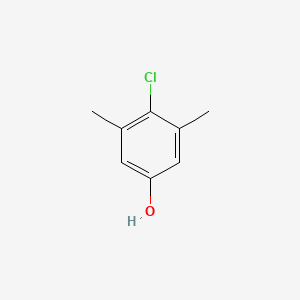

1. 2-chloro-5-hydroxy-1,3-dimethylbenzene
2. 3,5-dimethyl-4-chlorophenol
3. 4-chloro-3,5-dimethylphenol
4. 4-chloro-3,5-dimethylphenol Sulfonate
5. Chloroxylenol, Potassium Salt
6. Chloroxylenol, Sodium Salt
7. Dettol
8. Ice-o-derm
9. Micro-guard
10. P-chloro-m-xylenol
11. Parachlorometaxylenol
12. Pcmx
13. Sween Prep
1. 4-chloro-3,5-dimethylphenol
2. 88-04-0
3. Dettol
4. 4-chloro-3,5-xylenol
5. Pcmx
6. P-chloro-m-xylenol
7. Benzytol
8. 2-chloro-m-xylenol
9. 4-chloro-m-xylenol
10. Phenol, 4-chloro-3,5-dimethyl-
11. Ottasept
12. Desson
13. Espadol
14. Chloro-xylenol
15. Parachlorometaxylenol
16. Ottasept Extra
17. Husept Extra
18. P-chloro-3,5-xylenol
19. Willenol V
20. 3,5-dimethyl-4-chlorophenol
21. Septiderm-hydrochloride
22. Chloroxylenolum
23. Cloroxilenol
24. 2-chloro-5-hydroxy-m-xylene
25. Dettol, Liquid Antiseptic
26. Nipacide Mx
27. Parametaxylenol
28. Rba 777
29. 2-chloro-5-hydroxy-1,3-dimethylbenzene
30. 4-chloro-1-hydroxy-3,5-dimethylbenzene
31. 3,5-xylenol, 4-chloro-
32. Nsc 4971
33. Parachlorometoxylenol
34. 4-chloro-3,5-dimethyl-phenol
35. Nsc-4971
36. 4-chloro-3, 5-xylenol
37. Chlorxylenolum
38. Chebi:34393
39. Nsc4971
40. 0f32u78v2q
41. 4-chloro-3,5-dimethylphenol;pcmx
42. Ncgc00094614-03
43. Clorossilenolo
44. Dsstox_cid_12316
45. Dsstox_rid_78913
46. Dsstox_gsid_32316
47. Caswell No. 218
48. Clorossilenolo [dcit]
49. Vionexus
50. Cas-88-04-0
51. Cloroxilenol [inn-spanish]
52. Camel (pesticide)
53. Chloroxylenolum [inn-latin]
54. Hsdb 7427
55. Einecs 201-793-8
56. Epa Pesticide Chemical Code 086801
57. Brn 1862539
58. Ayrtol
59. Unii-0f32u78v2q
60. Ai3-08632
61. 5-dimethylphenol
62. Nipacide Px
63. Chloroxylenol(usan
64. Chloroxylenol [usan:usp:inn:ban]
65. Chloroxylenol-[d6]
66. Spectrum_000138
67. 3, 4-chloro-
68. M-xylenol, 4-chloro-
69. Para-chloro-meta-xylenol
70. Spectrum2_000136
71. Spectrum3_000344
72. Spectrum4_000281
73. Spectrum5_000713
74. Chloroxylenol (usp/inn)
75. Chloroxylenol [ii]
76. Chloroxylenol [mi]
77. Chloroxylenol [inn]
78. 4-chloro-3,5dimethylphenol
79. Chloroxylenol [hsdb]
80. Chloroxylenol [inci]
81. Chloroxylenol [usan]
82. Schembl34163
83. Bspbio_002007
84. Chloroxylenol [vandf]
85. Kbiogr_000802
86. Kbioss_000598
87. P-chloro-3,5-dimethylphenol
88. Mls000028592
89. Bidd:er0218
90. Chloroxylenol [mart.]
91. Divk1c_000801
92. Spectrum1500182
93. Spbio_000212
94. Wln: Qr Dg C1 E1
95. Chloroxylenol [usp-rs]
96. Chloroxylenol [who-dd]
97. 3, 5-dimethyl-4-chlorophenol
98. Chembl398440
99. Zinc1132
100. Dtxsid0032316
101. Hms502i03
102. Kbio1_000801
103. Kbio2_000598
104. Kbio2_003166
105. Kbio2_005734
106. Kbio3_001227
107. Ninds_000801
108. Hms1920k19
109. Hms2091c22
110. Hms2233n06
111. Hms3369i18
112. Para Chloro Meta Xylenol (pcmx)
113. Pharmakon1600-01500182
114. 4-chloro-3,5-dimethylphenol Purum
115. Hy-b1414
116. Parachlorometoxylenol [vandf]
117. Tox21_111305
118. Tox21_302047
119. Ac-265
120. Ccg-38943
121. Chloroxylenol [usp Monograph]
122. Mfcd00002324
123. Nsc756683
124. S4518
125. Stl183324
126. 4-chloro-3,5-dimethylphenol, 99%
127. Akos009159132
128. Tox21_111305_1
129. Cs-4912
130. Db11121
131. Nsc-756683
132. Idi1_000801
133. Ncgc00094614-01
134. Ncgc00094614-02
135. Ncgc00094614-04
136. Ncgc00094614-06
137. Ncgc00094614-07
138. Ncgc00255257-01
139. Ls-13415
140. Smr000059157
141. Sbi-0051310.p003
142. Db-028803
143. Ft-0618059
144. A16004
145. D03473
146. Ab00051942_07
147. A842444
148. Q426460
149. Sr-01000778359
150. Sr-01000778359-2
151. 4-chloro-3,5-dimethylphenol, Purum, >=98.0% (t)
152. 4-chloro-3,5-xylenol, 4-chloro-sym-m-xylenol, Pcmx
153. Brd-k17223896-001-02-7
154. Brd-k17223896-001-06-8
155. F0001-2183
156. Z1235963354
157. Chloroxylenol, British Pharmacopoeia (bp) Reference Standard
158. Chloroxylenol, United States Pharmacopeia (usp) Reference Standard
159. Chloroxylenol, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 156.61 g/mol |
|---|---|
| Molecular Formula | C8H9ClO |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 156.0341926 g/mol |
| Monoisotopic Mass | 156.0341926 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 104 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antibacterial; antiseptic (topical and urinary).
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 375
(VET): Antiseptic (topical).
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 375
The predominant medical applications for which chloroxylenol is formally indicated for therapeutic use is as an application to the skin for use in cuts, bites, stings, abrasions, and for use as antiseptic hand cleaner.
Chloroxylenol is a substituted phenol which has been widely used for many years as an ingredient of antiseptic and disinfectant products intended for external use. It is known to be bactericidal in low concentration to a wide range of Gram positive and Gram negative bacteria.
Schistosomicides
Agents that act systemically to kill adult schistosomes. (See all compounds classified as Schistosomicides.)
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
Disinfectants
Substances used on inanimate objects that destroy harmful microorganisms or inhibit their activity. Disinfectants are classed as complete, destroying SPORES as well as vegetative forms of microorganisms, or incomplete, destroying only vegetative forms of the organisms. They are distinguished from ANTISEPTICS, which are local anti-infective agents used on humans and other animals. (From Hawley's Condensed Chemical Dictionary, 11th ed) (See all compounds classified as Disinfectants.)
D08AE05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D08 - Antiseptics and disinfectants
D08A - Antiseptics and disinfectants
D08AE - Phenol and derivatives
D08AE05 - Chloroxylenol
Absorption
No chloroxylenol was detected in the blood following the dermal administration of 2 g of p-chloroxylenol in an ethanol/olive oil vehicle in human subjects. After a dose of 5 g, only traces were found, after 8 g, 1 mg % (1 mg/dL) was found in the blood after 3 hours, and 4 mg % (4 mg/dL) after 24 hours. After a dose of 20 g, 4 mg % (4 mg/dL) was measured after half an hour, and 1 mg % (1 mg/dL) was present at 72 hours. For antiseptic purposes, chloroxylenol is considered to be well-absorbed when applied to the skin.
Route of Elimination
The major route of excretion is likely in urine, although some amounts may be found in bile and traces in exhaled air.
Volume of Distribution
The only data available regarding the volume of distribution of chloroxylenol is the mean Vss of 22.45 L determined after 200 mg intravenous single dose of chloroxylenol was administered to healthy mongrel dog subjects.
Clearance
The only data available regarding the clearance of chloroxylenol is the mean clearance rate of 13.76 L/hr following a 200 mg intravenous single dose of the substance into healthy mongrel dog subjects. Moreover, in another study, when 8 g of chloroxylenol was administered dermal on a human subject in an alcohol/glycerin vehicle, 11% was excreted in 48 hours.
Mongrel dogs received iv and oral single doses of 200 and 2000 mg 4-chloro-3,5-xylenol, respectively. Low range of absorption was noted. Kidneys were not the major route for rapid elimination of unchanged 4-chloro-3,5-xylenol.
Sheftel, V.O.; Indirect Food Additives and Polymers. Migration and Toxicology. Lewis Publishers, Boca Raton, FL. 2000., p. 880
Tests of a 25 percent solution of chloroxylenol with Sprague-Dawley rats demonstrated the chemical was practically all eliminated in the first 24 hours, mostly in the urine, with small amounts in feces, after oral or dermal exposure. Following dermal exposure, about half of the material was not absorbed. High concentrations were found in the tissues of the kidney, which indicates excretion in urine. Concentrations in the lungs indicates some elimination in expired air.
USEPA; Reregistration Eligibility Decision (RED) Database for Chloroxylenol (88-04-0). EPA 738-R-94-032 (September 1994). Available from, as of January 31, 2006: https://www.epa.gov/pesticides/reregistration/status.htm
In a study /with/ beagle dogs dosed orally excreted virtually all of the chloroxylenol in their urine within 24 hours. A small amount was present in feces, but essentially none remained in any tissue.
USEPA; Reregistration Eligibility Decision (RED) Database for Chloroxylenol (88-04-0). EPA 738-R-94-032 (September 1994). Available from, as of January 31, 2006: https://www.epa.gov/pesticides/reregistration/status.htm
The pharmacokinetic and metabolic profile of p-chloro-m-xylenol (PCMX) was studied in healthy mongrel dogs after intravenous and oral administration of single doses of 200 and 2000 mg of PCMX, respectively. ... The mean half-life and mean residence time were 1.84 and 1.69 hr. respectively. The apparent volume of distribution at steady state was estimated to be 22.4 liters, and the plasma clearance was 14.6 liters/hr. The bioavailability of PCMX was 21%. ... PCMX's metabolite data show that a presystemic elimination process (first-pass effect) is also occurring. PCMX plasma concentrations after intravenous administration of 500-, 200-, and 100-mg doses were found to be proportional to the dose given.
Dorantes A, Stavchansky S; Pharm Res (NY) 9 (5): 677-682 (1992)
Certain animal studies have shown that following dermal application of chloroxylenol, that the absorption was rapid with a Cmax = 1-2 hours, and that the administered substance was excreted via the kidney with almost complete elimination within 24 hours. The primary metabolites discovered in the excreted urine were glucuronides and sulfates. Some chloroxylenol monographs liken its pharmacokinetic profile to that of another antiseptic - triclosan - which is rapidly excreted in the urine also as a glucuronide metabolite, as observed in the human model. Moreover, In one human subject administered 5 mg intragluteally, 14% was excreted with glucuronic acid and 17% with sulfuric acid at 3 days. Any chloroxylenol absorbed into the body is likely extensively metabolized by the liver and rapidly excreted, mainly in the urine, as sulphate and glucuronide conjugates.
Mongrel dogs received iv and oral single doses of 200 and 2000 mg 4-chloro-3,5-xylenol, respectively. ...Main metabolites found in the urine were glucuronides and sulfates.
Sheftel, V.O.; Indirect Food Additives and Polymers. Migration and Toxicology. Lewis Publishers, Boca Raton, FL. 2000., p. 880
One study estimated the mean terminal half-life and mean residence time after a 200 mg intravenous single dose of chloroxylenol in healthy mongrel dog subjects to be 1.7 and 1.69 hours, respectively. Alternatively, some product monographs liken chloroxylenol to a similar liquid antiseptic, triclosan, whose calculated urinary excretion half-life in man is approximately 10 hours.
As a phenol antiseptic, it is believed that the hydroxyl -OH groups of the chloroxylenol molecule binds to certain proteins on the cell membrane of bacteria, and disrupts the membrane so as to allow the contents of the bacterial cell to leak out. This allows chloroxylenol to enter the bacterial cell to bind further with more proteins and enzymes to disable the cell's functioning. At particularly high concentrations of chloroxylenol, the protein and nucleic acid content of targeted bacterial cells become coagulated and cease to function, leading to rapid cell death.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
81
PharmaCompass offers a list of Chloroxylenol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Chloroxylenol manufacturer or Chloroxylenol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Chloroxylenol manufacturer or Chloroxylenol supplier.
PharmaCompass also assists you with knowing the Chloroxylenol API Price utilized in the formulation of products. Chloroxylenol API Price is not always fixed or binding as the Chloroxylenol Price is obtained through a variety of data sources. The Chloroxylenol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Chloroxylenol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Chloroxylenol, including repackagers and relabelers. The FDA regulates Chloroxylenol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Chloroxylenol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Chloroxylenol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Chloroxylenol supplier is an individual or a company that provides Chloroxylenol active pharmaceutical ingredient (API) or Chloroxylenol finished formulations upon request. The Chloroxylenol suppliers may include Chloroxylenol API manufacturers, exporters, distributors and traders.
click here to find a list of Chloroxylenol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Chloroxylenol DMF (Drug Master File) is a document detailing the whole manufacturing process of Chloroxylenol active pharmaceutical ingredient (API) in detail. Different forms of Chloroxylenol DMFs exist exist since differing nations have different regulations, such as Chloroxylenol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Chloroxylenol DMF submitted to regulatory agencies in the US is known as a USDMF. Chloroxylenol USDMF includes data on Chloroxylenol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Chloroxylenol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Chloroxylenol suppliers with USDMF on PharmaCompass.
A Chloroxylenol written confirmation (Chloroxylenol WC) is an official document issued by a regulatory agency to a Chloroxylenol manufacturer, verifying that the manufacturing facility of a Chloroxylenol active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Chloroxylenol APIs or Chloroxylenol finished pharmaceutical products to another nation, regulatory agencies frequently require a Chloroxylenol WC (written confirmation) as part of the regulatory process.
click here to find a list of Chloroxylenol suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Chloroxylenol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Chloroxylenol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Chloroxylenol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Chloroxylenol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Chloroxylenol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Chloroxylenol suppliers with NDC on PharmaCompass.
Chloroxylenol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Chloroxylenol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Chloroxylenol GMP manufacturer or Chloroxylenol GMP API supplier for your needs.
A Chloroxylenol CoA (Certificate of Analysis) is a formal document that attests to Chloroxylenol's compliance with Chloroxylenol specifications and serves as a tool for batch-level quality control.
Chloroxylenol CoA mostly includes findings from lab analyses of a specific batch. For each Chloroxylenol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Chloroxylenol may be tested according to a variety of international standards, such as European Pharmacopoeia (Chloroxylenol EP), Chloroxylenol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Chloroxylenol USP).

