
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
Europe
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
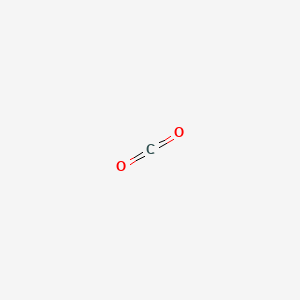

1. Anhydride, Carbonic
2. Carbonic Anhydride
3. Dioxide, Carbon
1. Carbonic Anhydride
2. Dry Ice
3. 124-38-9
4. Carbonic Acid Gas
5. Carbonic Acid Anhydride
6. Carbonica
7. Carbon Oxide, Di-
8. Methanedione
9. Kohlendioxyd
10. Kohlensaure
11. Khladon 744
12. Co2
13. Anhydride Carbonique
14. Dioxomethane
15. Cardice
16. Drikold
17. Hsdb 516
18. R 744
19. Un1013
20. Un1845
21. Un2187
22. Carbon Oxide (co2)
23. Dry Ice (solid Form)
24. Ins No.290
25. E-290
26. Chebi:16526
27. Ins-290
28. 142m471b3j
29. E290
30. After-damp
31. Aer Fixus
32. 18923-20-1
33. Kohlensaure [german]
34. Caswell No. 163
35. Kohlendioxyd [german]
36. Dioxido De Carbono
37. Dioxyde De Carbone
38. Dioxyde De Carbone [french]
39. Dioxido De Carbono [spanish]
40. Anhydride Carbonique [french]
41. Carbon Dioxide [usp]
42. Einecs 204-696-9
43. Epa Pesticide Chemical Code 016601
44. Carbondioxide
45. Dioxidocarbon
46. Epoxyketone
47. Dricold
48. Carbon Dioxid
49. Dry-ice
50. Dioxomethane #
51. Methane, Dioxo-
52. Unii-142m471b3j
53. Carbon Dioxide, Refrigerated Liquid
54. Carbonic Acid, Gas
55. Makr Carbon Dioxide
56. Carbon-12 Dioxide
57. Carbon Dioxide (tn)
58. Carbon-dioxide
59. Carbon Dioxide [ii]
60. Carbon Dioxide [mi]
61. Carbon Dioxide [fcc]
62. Carbon Dioxide [jan]
63. Carbon Dioxide (jp17/usp)
64. Carbon Dioxide [hsdb]
65. Carbon Dioxide [inci]
66. Carbon Dioxide, >=99.8%
67. Carbon Dioxide [vandf]
68. Carbon Dioxide [mart.]
69. Carbon Dioxide [who-dd]
70. Chembl1231871
71. Dtxsid4027028
72. Bdbm10856
73. Carbon Dioxide, Solid Or Dry Ice
74. [co2]
75. Carbon Dioxide [green Book]
76. Carbon Dioxide [ep Impurity]
77. Nsc-81688
78. Carbon Dioxide [ep Monograph]
79. Carbon Dioxide [usp Monograph]
80. Db09157
81. Un 1013
82. Un 1845
83. Un 2187
84. Carbon Dioxide, Puriss., >=99.998%
85. E 290
86. Ft-0690212
87. Q1997
88. C00011
89. Carbon Dioxide [un1013] [nonflammable Gas]
90. Carbon Dioxide, Messer(r) Cangas, 99.995%
91. D00004
92. Carbon Dioxide, Solid Or Dry Ice [un1845] [class 9]
93. Carbon Dioxide (99.8%), Cylinder Of 14 L, Analytical Standard
94. Carbon Dioxide (99.8%), Cylinder Of 48 L, Analytical Standard
95. Carbon Dioxide, Refrigerated Liquid [un2187] [nonflammable Gas]
| Molecular Weight | 44.009 g/mol |
|---|---|
| Molecular Formula | CO2 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 43.989829239 g/mol |
| Monoisotopic Mass | 43.989829239 g/mol |
| Topological Polar Surface Area | 34.1 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 18.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
CO2 can be used to flood the surgical field during cardiac surgery. Because of its density, carbon dioxide displaces the air surrounding the open heart so that any gas bubbles trapped in the heart are carbon dioxide rather than insoluble nitrogen. Similarly, CO2 is used to de-bubble cardiopulmonary bypass and extracorporeal membrane oxygenation (ECMO) circuits. It is used to adjust pH during cardiopulmonary bypass procedures when a patient is cooled.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 558
Hypocarbia results in ... decreased blood pressure and vasoconstriction in skin, intestine, brain, kidney, and heart. These actions are exploited clinically in the use of hyperventilation to diminish intracranial hypertension.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 557
CO2 is used for insufflation during endoscopic procedures (e.g., laparoscopic surgery) because it is highly soluble and does not support combustion. Inadvertent gas emboli thus are dissolved and eliminated more easily via the respiratory system.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 558
Medication (Vet): wart destruction
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 82
For more Therapeutic Uses (Complete) data for Carbon dioxide (8 total), please visit the HSDB record page.
In patients who are hypoventilating from /CNS depressants/ or anesthetics, increasing PCO2 may result in further CNS depression, which in turn may worsen the respiratory depression.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 558
Since carbon dioxide is the most potent cerebrovascular dilator known, it should not be used in patients with increased intracranial pressure, intracranial bleeding, expanding lesions, head injury, or in those in coma.
American Medical Association, Council on Drugs. AMA Drug Evaluations. 2nd ed. Acton, Mass.: Publishing Sciences Group, Inc., 1973., p. 507
The inhalation of high concentrations of carbon dioxide (about 50%) produces marked cortical and subcortical depression of a type similar to that produced by anesthetic agents.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 558
Carbon dioxide is commonly used as an insufflation gas for minimal invasive surgery (laparoscopy, endoscopy, and arthroscopy) to enlarge and stabilize body cavities to provide better visibility of the surgical area. It has been used also in cryotherapy and as respiratory stimulant before and after anesthesia. It could be used also in expansion of blood vessels if required, to increase carbon dioxide level after rapid breathing, and to stimulate breathing after a period of nonbreathing.
Data not found.
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AN - Medical gases
V03AN02 - Carbon dioxide
Absorption
Data not found.
Route of Elimination
Data not found.
Volume of Distribution
Data not found.
Clearance
Data not found.
Carbon dioxide is excreted by the lungs and, in the form of bicarbonate ion, by the kidney, intestine and skin.
Osol, A., and R. Pratt. (eds.). The United States Dispensatory. 27th ed. Philadelphia: J.B. Lippincott, 1973., p. 231
Carbon dioxide is produced by metabolism at approximately the same rate as O2 is consumed. At rest, this value is about 3 mL/kg per minute, but it may increase dramatically with heavy exercise. Carbon dioxide diffuses readily from the cells into the blood, where it is carried partly as bicarbonate ion (HCO3-), partly in chemical combination with hemoglobin and plasma proteins, and partly in solution at a partial pressure of about 6 kPa (46 mmHg) in mixed venous blood. CO2 is transported to the lung, where it is normally exhaled at the rate it is produced, leaving a partial pressure of about 5.2 kPa (40 mmHg) in the alveoli and in arterial blood.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 557
Gases and vapors known to be absorbed (or excreted) by the skin include ... carbon dioxide. ... About 2.7% of the carbon dioxide produced is excreted by /the skin/.
Hayes, W.J., Jr., E.R. Laws Jr., (eds.). Handbook of Pesticide Toxicology Volume 1. General Principles. New York, NY: Academic Press, Inc., 1991., p. 139
Not metabolized.
Carbon dioxide is produced by the body's metabolism and is always present in the body at about 6% concentration. An average adult human will produce more than 500 g of carbon dioxide daily under resting conditions, and will produce much more when active. Additional carbon dioxide has several effects on the body, and responses are immediate. It stimulates breathing, which exhales the carbon dioxide carried to the lungs from the cells by the bloodstream. An increase in carbon dioxide concentration stimulates the heart rate, increases the blood pressure, increases adrenalin flow, and relaxes the vascular smooth muscles. In addition, carbon dioxide reacts with water in the body to form carbonic acid, which dissociates to hydrogen ion and bicarbonate. An increase in carbon dioxide in the body increases acidity, and then the kidneys act to restore normal acidity.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Carbon and Carbon Dioxide pp.8-9 EPA-4019 (September 1991). Available from, as of November 23, 2009: https://www.epa.gov/pesticides/reregistration/status.htm
Perturbation of mitochondrial metabolism, oxidative phosphorylation or Krebs cycle affects embryogenesis. These studies assess the effects of altering pyruvate metabolism in 3-5 somite mouse embryos in whole embryo culture. ... To establish that pyruvate is metabolized during organogenesis the rates of (14)C-carbon dioxide production from 3-(14)C-pyruvate by conceptuses in vitro were measured on days 9-12. The rates of carbon dioxide production incr with incr gestational age. ... The rate of carbon dioxide production from pyruvate by day 10 yolk sac was 10 times greater than that by the embryo proper. Fluoroacetate produced a time and concn dependent reduction in carbon dioxide production in day 10 conceptuses. These studies demonstrate that pyruvate is metabolized by Krebs cycle during organogenesis. alpha-Cyano-4-hydroxycinnamate inhibits transport of pyruvate from cytosol to mitochondria. Embryos exposed to alpha-Cyano-4-hydroxycinnamate exhibited neural tube defects (11/12 at 1,000 uM). Thus, alterations of utilization of pyruvate is teratogenic to cultured embryos. Pyruvate dehydrogenase is inhibited via phosphorylation by E1-kinase. Dichloroacetate inhibits e1-kinase resulting incr pyruvate dehydrogenase activity and incr metabolism of pyruvate by Krebs cycle. Dichloroacetate produced neural tube defects in 0/12 embryos at 100 uM and 6/15 embryos at 500 uM. ... Pyruvate is metabolized during organogenesis and that proper regulation of pyruvate is metabolized during organogenesis and that proper regulation of pyruvate transport and metabolism is essential for normal development.
Hunter ES; Teratol 49 (5): 394 (1994)
Data not found.
Data not found.
Supercritical carbon dioxide possesses germicide (bactericide and sporicide) effect. Despite of the fact, that this effect is used in industrial sterilization processes, the sterilization mechanism at molecular level is unclear. Our hypotheses can provide a molecular-biological explanation for the phenomenon. We believe that in supercritical state CO(2) reacts competitively with Met-tRNA(fMet), the formation rate and the amount of formyl-methionyl-tRNA (fMet-tRNA(fMet)) will be diminished by irreversible substrate consumption. The fMet-tRNA(fMet) possesses a key role in prokaryotic protein synthesis, being almost exclusively the initiator aminoacyl-tRNA. The formed carbamoyl-methionyl-tRNA (cMet-tRNA(fMet)), probably stable only under pressure and high CO(2) concentration, is stabilized by forming a ternary molecular complex with the GTP-form of the translational initiation factor 2 (GTP-IF2). This complex is unable to dissociate from preinitiation 70S ribosomal complex because of strong polar binding between the protein C-2 domain and the modified initiator aminoacyl-tRNA. The IF2-fMet-tRNA(fMet)-blocked 70S ribosomal preinitiation complex does not decompose following the GTP hydrolysis, becoming unable to synthesize proteins. The death of the microbial cell is caused by inhibition of the protein synthesis and energetic depletion. Moreover, we propose a possible mechanism for the accumulation of cMet-tRNA(fMet) in the bacterial cell. Since the translational process is an important target for antibiotics, the proposed mechanism could be a work hypothesis for discovery of new antibiotics. Made by high conservative character of prokaryotic translation initiation, the proposed IF2 pathway deterioration strategy may conduct to obtaining selective (with low mammalian toxicity) antimicrobials and at the same time, with reduced possibility of the drug resistance development.
PMID:19765910 Andras CD et al; Med Hypotheses 74 (2): 325-9 (2010)
... Well fed C. elegans (roundworm) avoid CO2 levels above 0.5%. Animals can respond to both absolute CO2 concentrations and changes in CO2 levels within seconds. Responses to CO2 do not reflect avoidance of acid pH but appear to define a new sensory response. Sensation of CO2 is promoted by the cGMP-gated ion channel subunits TAX-2 and TAX-4, but other pathways are also important. Robust CO2 avoidance in well fed animals requires inhibition of the DAF-16 forkhead transcription factor by the insulin-like receptor DAF-2. Starvation, which activates DAF-16, strongly suppresses CO2 avoidance. Exposure to hypoxia (<1% O2) also suppresses CO2 avoidance via activation of the hypoxia-inducible transcription factor HIF-1. The npr-1 215V allele of the naturally polymorphic neuropeptide receptor npr-1, besides inhibiting avoidance of high ambient O2 in feeding C. elegans, also promotes avoidance of high CO2. C. elegans integrates competing O2 and CO2 sensory inputs so that one response dominates. Food and allelic variation at NPR-1 regulate which response prevails. These results suggest that multiple sensory inputs are coordinated by C. elegans to generate different coherent foraging strategies.
PMID:18524954 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2410288 Bretscher AJ et al; Proc Nat Acad Sci USA 105 (23): 8044-9 (2008)
... Adult Caenorhabditis elegans (roundworm) display an acute avoidance response upon exposure to CO2 that is characterized by the cessation of forward movement and the rapid initiation of backward movement. This response is mediated by a cGMP signaling pathway that includes the cGMP-gated heteromeric channel TAX-2/TAX-4. CO2 avoidance is modulated by multiple signaling molecules, including the neuropeptide Y receptor NPR-1 and the calcineurin subunits TAX-6 and CNB-1. Nutritional status also modulates CO2 responsiveness via the insulin and TGFbeta signaling pathways. CO2 response is mediated by a neural circuit that includes the BAG neurons, a pair of sensory neurons of previously unknown function. TAX-2/TAX-4 function in the BAG neurons to mediate acute CO2 avoidance. ... C. elegans senses and responds to CO2 using multiple signaling pathways and a neural network that includes the BAG neurons and this response is modulated by the physiological state of the worm.
PMID:18524955 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2430355 Hallem EA, Sternberg PW; Proc Nat Acad Sci USA 105 (23): 8038-43 (2008)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
92
PharmaCompass offers a list of Carbon Dioxide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Carbon Dioxide manufacturer or Carbon Dioxide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Carbon Dioxide manufacturer or Carbon Dioxide supplier.
PharmaCompass also assists you with knowing the Carbon Dioxide API Price utilized in the formulation of products. Carbon Dioxide API Price is not always fixed or binding as the Carbon Dioxide Price is obtained through a variety of data sources. The Carbon Dioxide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Carbon Dioxide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Carbon Dioxide, including repackagers and relabelers. The FDA regulates Carbon Dioxide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Carbon Dioxide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Carbon Dioxide supplier is an individual or a company that provides Carbon Dioxide active pharmaceutical ingredient (API) or Carbon Dioxide finished formulations upon request. The Carbon Dioxide suppliers may include Carbon Dioxide API manufacturers, exporters, distributors and traders.
click here to find a list of Carbon Dioxide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Carbon Dioxide DMF (Drug Master File) is a document detailing the whole manufacturing process of Carbon Dioxide active pharmaceutical ingredient (API) in detail. Different forms of Carbon Dioxide DMFs exist exist since differing nations have different regulations, such as Carbon Dioxide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Carbon Dioxide DMF submitted to regulatory agencies in the US is known as a USDMF. Carbon Dioxide USDMF includes data on Carbon Dioxide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Carbon Dioxide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Carbon Dioxide suppliers with USDMF on PharmaCompass.
A Carbon Dioxide CEP of the European Pharmacopoeia monograph is often referred to as a Carbon Dioxide Certificate of Suitability (COS). The purpose of a Carbon Dioxide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Carbon Dioxide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Carbon Dioxide to their clients by showing that a Carbon Dioxide CEP has been issued for it. The manufacturer submits a Carbon Dioxide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Carbon Dioxide CEP holder for the record. Additionally, the data presented in the Carbon Dioxide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Carbon Dioxide DMF.
A Carbon Dioxide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Carbon Dioxide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Carbon Dioxide suppliers with CEP (COS) on PharmaCompass.
Carbon Dioxide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Carbon Dioxide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Carbon Dioxide GMP manufacturer or Carbon Dioxide GMP API supplier for your needs.
A Carbon Dioxide CoA (Certificate of Analysis) is a formal document that attests to Carbon Dioxide's compliance with Carbon Dioxide specifications and serves as a tool for batch-level quality control.
Carbon Dioxide CoA mostly includes findings from lab analyses of a specific batch. For each Carbon Dioxide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Carbon Dioxide may be tested according to a variety of international standards, such as European Pharmacopoeia (Carbon Dioxide EP), Carbon Dioxide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Carbon Dioxide USP).

