
Synopsis
Synopsis
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
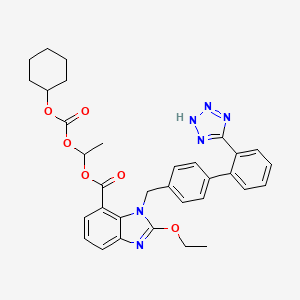

1. 1-(cyclohexylocarbonyloxy)ethyl-2-ethoxy-1-(2'-(1h-tetrazol-5-yl)biphenyl-4-yl)-1h-benzimidazole-7-carboxylate
2. 1h-benzimidazolium, 7-carboxy-1-(2-((cyclohexylcarbonyl)oxy)ethyl)-2-ethoxy-1-(2'-(1h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)-, Hydroxide, Inner Salt, (+-)-
3. Amias
4. Atacand
5. Blopress
6. Kenzen
7. Parapres
8. Tcv 116
9. Tcv-116
1. 145040-37-5
2. Atacand
3. Tcv-116
4. Amias
5. Parapres
6. Kenzen
7. Candesartan Cilextil
8. Tcv 116
9. Candesartancilexetil
10. Candesartan Cilexetil [usan]
11. Nsc-758697
12. Chembl1014
13. Chebi:3348
14. 1-(((cyclohexyloxy)carbonyl)oxy)ethyl 1-((2'-(2h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate
15. R85m2x0d68
16. 1-(cyclohexyloxycarbonyloxy)ethyl 1-((2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate
17. 1-cyclohexyloxycarbonyloxyethyl 2-ethoxy-3-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylate
18. 1-(((cyclohexyloxy)carbonyl)oxy)ethyl 1-((2'-(1h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate
19. Candesartan 1-(((cyclohexyloxy)carbonyl)oxy)ethyl Ester
20. Candesartan 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl Ester
21. Racanda
22. Unii-r85m2x0d68
23. Tcy 116
24. Candesartan Hexetil
25. 1-cyclohexyloxycarbonyloxyethyl 2-ethoxy-3-[[4-[2-(1h-tetrazol-5-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylate
26. Atacand (tn)
27. Candesartan Cilexitil
28. Mfcd00871371
29. Spectrum_001707
30. Candesartan (cilexetil)
31. Dsstox_cid_239
32. Spectrum2_000485
33. Spectrum3_000996
34. Spectrum4_001124
35. Spectrum5_001462
36. Candesartan Celexetil Ester
37. Dsstox_rid_85567
38. Dsstox_gsid_20239
39. Schembl40831
40. Bspbio_002691
41. Candesartan Cilexetil- Bio-x
42. Kbiogr_001607
43. Kbioss_002187
44. 1h-benzimidazolium, 7-carboxy-1-(2-((cyclohexylcarbonyl)oxy)ethyl)-2-ethoxy-1-(2'-(1h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)-, Hydroxide, Inner Salt, (+-)-
45. Candesartan Cilexetil-[d11]
46. Mls004774127
47. Candesartan Cilexetil , 97%
48. Spectrum1504261
49. Spbio_000349
50. Gtpl8352
51. Candesartan Cilexetil (atacand)
52. Dtxsid5020239
53. Kbio2_002187
54. Kbio2_004755
55. Kbio2_007323
56. Kbio3_001911
57. Hms1922j09
58. Hms2093e20
59. Hms3651i08
60. Pharmakon1600-01504261
61. Candesartan Cilexetil (jp17/usp)
62. Candesartan Cilexetil [jan]
63. Bcp22050
64. Tox21_302202
65. Ac-204
66. Bdbm50318907
67. Candesartan Cilexetil [vandf]
68. Ccg-39530
69. Nsc758697
70. S2037
71. Stl451065
72. Candesartan Cilexetil [mart.]
73. Akos015894954
74. Akos015920180
75. Candesartan Cilexetil [usp-rs]
76. Candesartan Cilexetil [who-dd]
77. Ab07617
78. Am90293
79. Bcp9000480
80. Ccg-222334
81. Db00796
82. Ds-1302
83. Ks-1147
84. Nsc 758697
85. Candesartan Cilexetil, >=98% (hplc)
86. Ncgc00095123-01
87. Ncgc00095123-02
88. Ncgc00095123-03
89. Ncgc00095123-05
90. Ncgc00095123-10
91. Ncgc00095123-16
92. Ncgc00255218-01
93. (+-)-1-hydroxyethyl 2-ethoxy-1-(p-(o-1h-tetrazol-5-ylphenyl)benzyl)-7-benzimidazolecarboxylate, Cyclohexyl Carbonate (ester)
94. 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl 2-ethoxy-1-[[2'-(1h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1h-benzimidazole-7-carboxylate
95. 1-{[(cyclohexyloxy)carbonyl]oxy}ethyl 2-(ethyloxy)-1-{[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl}-1h-benzimidazole-7-carboxylate
96. 1h-benzimidazole-7-carboxylic Acid, 2-ethoxy-1-((2'-(1h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-, 1-(((cyclohexyloxy)carbonyl)oxy)ethyl Ester, (+-)-
97. 2-ethoxy-1-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1h-benzimidazole-7-carboxylic Acid 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl Ester
98. 2-ethoxy-3-[2'-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3h-benzoimidazole-4-carboxylic Acid 1-cyclohexyloxycarbonyloxy-ethyl Ester
99. Bc164274
100. Hy-17505
101. Smr003500784
102. Candesartan Cilexetil [orange Book]
103. Sbi-0206767.p001
104. Candesartan Cilexetil [ep Monograph]
105. Candesartan Cilexetil [usp Monograph]
106. Cas-145040-37-5
107. Ft-0602914
108. Sw220041-1
109. C07709
110. D00626
111. Ab01274805-01
112. Ab01274805_02
113. Ab01274805_03
114. 040c375
115. H212/91
116. L006257
117. Sr-05000001976
118. Camptothecine, Antibiotic For Culture Media Use Only
119. Q-200786
120. Sr-05000001976-1
121. Brd-a65671304-001-02-6
122. Brd-a65671304-001-03-4
123. Q27075664
124. Candesartan 1-(((cyclohexyloxy)carbonyl)oxy)ethyl Ester [mi]
125. Candesartan Cilexetil, European Pharmacopoeia (ep) Reference Standard
126. Candesartan Cilexetil, United States Pharmacopeia (usp) Reference Standard
127. Candesartan Cilexetil For Peak Identification, European Pharmacopoeia (ep) Reference Standard
128. Candesartan Cilexetil For System Suitability, European Pharmacopoeia (ep) Reference Standard
129. Candesartan Cilexetil, Pharmaceutical Secondary Standard; Certified Reference Material
130. (+/-)-1-(cyclohexyloxycarbonyloxy)ethyl 2-ethoxy-1-[2'-(1h-tetrazol-5-yl) Biphenyl-4-yl]methylbenzimidazole-7-carboxylate
131. (+/-)-1-(cyclohexyloxycarbonyloxy)ethyl 2-ethoxy-1-[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methylbenzimidazole-7-carboxylate
132. (+/-)1-hydroxyethyl 2-ethoxy-1-(p-(o-1h-tetrazol-5-ylphenyl)benzyl)-7-benzimidazolecarboxylate, Cyclohexyl Carbonate (ester)
133. 1-(((cyclohexyloxy)carbonyl)oxy)ethyl1-((2'-(2h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate
134. 1-(cyclohexyloxycarbonyloxy)ethyl-2-ethoxy-1-[[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl]benzimidazol-7-carboxylate
135. 1-{[(cyclohexyloxy)carbonyl]oxy}ethyl 2-ethoxy-1-{[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl}-1h-benzimidazole-7-carboxylate
136. 1-cyclohexyloxycarbonyloxyethyl 2-ethoxy-3-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl] Methyl]benzimidazole-4-carboxylate
137. 1-cyclohexyloxycarbonyloxyethyl 2-ethoxy-3-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylate.
138. 1h-benzimidazole-7-carboxylic Acid, 2-ethoxy-1-((2'-(1h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-, 1-(((cyclohexyloxy)carbonyl)oxy)ethyl Ester, (+/-)-
139. 1h-benzimidazole-7-carboxylic Acid, 2-ethoxy-1-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-, 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl Ester
140. 2-ethoxy-1-[2'-(1h-tetrazole-5-yl)-4-biphenylylmethyl]-1h-benzimidazole-7-carboxylic Acid 1-(cyclohexyloxycarbonyloxy)ethyl Ester
141. 2-ethoxy-3-[2''-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3h-benzoimidazole-4-carboxylic Acid 1-cyclohexyloxycarbonyloxy-ethyl Ester
142. 2-ethoxy-3-[2''-(2h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3h-benzoimidazole-4-carboxylic Acid 1-cyclohexyloxycarbonyloxy-ethyl Ester
143. 2-ethoxy-3-[2'-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3h-benzoimidazole-4-carboxylic Acid 1-cyclohe
1. Candesartan
| Molecular Weight | 610.7 g/mol |
|---|---|
| Molecular Formula | C33H34N6O6 |
| XLogP3 | 7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 13 |
| Exact Mass | 610.25398283 g/mol |
| Monoisotopic Mass | 610.25398283 g/mol |
| Topological Polar Surface Area | 143 Ų |
| Heavy Atom Count | 45 |
| Formal Charge | 0 |
| Complexity | 962 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Atacand |
| PubMed Health | Candesartan (By mouth) |
| Drug Classes | Angiotensin II Receptor Antagonist/Thiazide Combination |
| Drug Label | ATACAND (candesartan cilexetil), a prodrug, is hydrolyzed to candesartan during absorption from the gastrointestinal tract. Candesartan is a selective AT1 subtype angiotensin II receptor antagonist.Candesartan cilexetil, a nonpeptide, is chemically d... |
| Active Ingredient | Candesartan cilexetil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 32mg; 4mg; 16mg; 8mg |
| Market Status | Prescription |
| Company | Astrazeneca |
| 2 of 6 | |
|---|---|
| Drug Name | Atacand hct |
| PubMed Health | Candesartan/Hydrochlorothiazide (By mouth) |
| Active Ingredient | hydrochlorothiazide; Candesartan cilexetil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 32mg; 25mg; 16mg; 12.5mg |
| Market Status | Prescription |
| Company | Astrazeneca |
| 3 of 6 | |
|---|---|
| Drug Name | Candesartan cilexetil |
| Drug Label | Candesartan cilexetil, a prodrug, is hydrolyzed to candesartan during absorption from the gastrointestinal tract. Candesartan is a selective AT1 subtype angiotensin II receptor antagonist.Candesartan cilexetil, a nonpeptide, is chemically described a... |
| Active Ingredient | Candesartan cilexetil |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 32mg; 4mg; 16mg; 8mg |
| Market Status | Tentative Approval; Prescription |
| Company | Matrix Labs; Apotex; Sandoz |
| 4 of 6 | |
|---|---|
| Drug Name | Atacand |
| PubMed Health | Candesartan (By mouth) |
| Drug Classes | Angiotensin II Receptor Antagonist/Thiazide Combination |
| Drug Label | ATACAND (candesartan cilexetil), a prodrug, is hydrolyzed to candesartan during absorption from the gastrointestinal tract. Candesartan is a selective AT1 subtype angiotensin II receptor antagonist.Candesartan cilexetil, a nonpeptide, is chemically d... |
| Active Ingredient | Candesartan cilexetil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 32mg; 4mg; 16mg; 8mg |
| Market Status | Prescription |
| Company | Astrazeneca |
| 5 of 6 | |
|---|---|
| Drug Name | Atacand hct |
| PubMed Health | Candesartan/Hydrochlorothiazide (By mouth) |
| Active Ingredient | hydrochlorothiazide; Candesartan cilexetil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 32mg; 25mg; 16mg; 12.5mg |
| Market Status | Prescription |
| Company | Astrazeneca |
| 6 of 6 | |
|---|---|
| Drug Name | Candesartan cilexetil |
| Drug Label | Candesartan cilexetil, a prodrug, is hydrolyzed to candesartan during absorption from the gastrointestinal tract. Candesartan is a selective AT1 subtype angiotensin II receptor antagonist.Candesartan cilexetil, a nonpeptide, is chemically described a... |
| Active Ingredient | Candesartan cilexetil |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 32mg; 4mg; 16mg; 8mg |
| Market Status | Tentative Approval; Prescription |
| Company | Matrix Labs; Apotex; Sandoz |
May be used as a first line agent to treat uncomplicated hypertension, isolated systolic hypertension and left ventricular hypertrophy. May be used as a first line agent to delay progression of diabetic nephropathy. Candesartan may be also used as a second line agent in the treatment of congestive heart failure, systolic dysfunction, myocardial infarction and coronary artery disease in those intolerant of ACE inhibitors.
FDA Label
Diabetic retinopathy, Essential hypertension, Heart Failure
Diabetic retinopathy, Essential hypertension, Heart Failure
Candesartan cilexetil is an ARB prodrug that is rapidly converted to candesartan, its active metabolite, during absorption from the gastrointestinal tract. Candesartan confers blood pressure lowering effects by antagonizing the hypertensive effects of angiotensin II via the RAAS. RAAS is a homeostatic mechanism for regulating hemodynamics, water and electrolyte balance. During sympathetic stimulation or when renal blood pressure or blood flow is reduced, renin is released from granular cells of the juxtaglomerular apparatus in the kidneys. Renin cleaves circulating angiotensinogen to angiotensin I, which is cleaved by angiotensin converting enzyme (ACE) to angiotensin II. Angiotensin II increases blood pressure by increasing total peripheral resistance, increasing sodium and water reabsorption in the kidneys via aldosterone secretion, and altering cardiovascular structure. Angiotensin II binds to two receptors: type-1 angiotensin II receptor (AT1) and type-2 angiotensin II receptor (AT2). AT1 is a G-protein coupled receptor (GPCR) that mediates the vasoconstrictive and aldosterone-secreting effects of angiotensin II. Studies performed in recent years suggest that AT2 antagonizes AT1-mediated effects and directly affects long-term blood pressure control by inducing vasorelaxation and increasing urinary sodium excretion. Angiotensin receptor blockers (ARBs) are non-peptide competitive inhibitors of AT1. ARBs block the ability of angiotensin II to stimulate pressor and cell proliferative effects. Unlike ACE inhibitors, ARBs do not affect bradykinin-induced vasodilation. The overall effect of ARBs is a decrease in blood pressure.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Angiotensin II Type 1 Receptor Blockers
Agents that antagonize ANGIOTENSIN II TYPE 1 RECEPTOR. Included are ANGIOTENSIN II analogs such as SARALASIN and biphenylimidazoles such as LOSARTAN. Some are used as ANTIHYPERTENSIVE AGENTS. (See all compounds classified as Angiotensin II Type 1 Receptor Blockers.)
Absorption
Following administration of the candesartan cilexetil prodrug, the absolute bioavailability of candesartan was estimated to be 15%. Food with a high fat content has no effect on the bioavailability of candesartan from candesartan cilexetil.
Route of Elimination
When candesartan is administered orally, about 26% of the dose is excreted unchanged in urine. Candesartan is mainly excreted unchanged in urine and feces (via bile).
Volume of Distribution
0.13 L/kg
Clearance
0.37 mL/min/kg
The prodrug candesartan cilexetil undergoes rapid and complete ester hydrolysis in the intestinal wall to form the active drug, candesartan. Elimination of candesartan is primarily as unchanged drug in the urine and, by the biliary route, in the feces. Minor hepatic metabolism of candesartan (<20%) occurs by O-deethylation via cytochrome P450 2C9 to form an inactive metabolite. Candesartan undergoes N-glucuronidation in the tetrazole ring by uridine diphosphate glucuronosyltransferase 1A3 (UGT1A3). O-glucuronidation may also occur. 75% of candesartan is excreted as unchanged drug in urine and feces.
Approximately 9 hours.
Candesartan selectively blocks the binding of angiotensin II to AT1 in many tissues including vascular smooth muscle and the adrenal glands. This inhibits the AT1-mediated vasoconstrictive and aldosterone-secreting effects of angiotensin II and results in an overall decrease in blood pressure. Candesartan is greater than 10,000 times more selective for AT1 than AT2. Inhibition of aldosterone secretion may increase sodium and water excretion while decreasing potassium excretion.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

Product Code : JM08S04-1504WT
Classification : DrugsC Metabolites
Product Characteristics : R100702O
Category :
Description :

Product Code : TO12S03-0502WT
Classification : DrugsC Metabolites
Product Characteristics : U110801J
Category :
Description :

Product Code : JM08S04-1504WT
Classification : DrugsC Metabolites
Product Characteristics : R100702P
Category :
Description :

ABOUT THIS PAGE
32
PharmaCompass offers a list of Candesartan Cilexetil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Candesartan Cilexetil manufacturer or Candesartan Cilexetil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Candesartan Cilexetil manufacturer or Candesartan Cilexetil supplier.
PharmaCompass also assists you with knowing the Candesartan Cilexetil API Price utilized in the formulation of products. Candesartan Cilexetil API Price is not always fixed or binding as the Candesartan Cilexetil Price is obtained through a variety of data sources. The Candesartan Cilexetil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Candesartan Cilexetil manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Candesartan Cilexetil, including repackagers and relabelers. The FDA regulates Candesartan Cilexetil manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Candesartan Cilexetil API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Candesartan Cilexetil manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Candesartan Cilexetil supplier is an individual or a company that provides Candesartan Cilexetil active pharmaceutical ingredient (API) or Candesartan Cilexetil finished formulations upon request. The Candesartan Cilexetil suppliers may include Candesartan Cilexetil API manufacturers, exporters, distributors and traders.
click here to find a list of Candesartan Cilexetil suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Candesartan Cilexetil DMF (Drug Master File) is a document detailing the whole manufacturing process of Candesartan Cilexetil active pharmaceutical ingredient (API) in detail. Different forms of Candesartan Cilexetil DMFs exist exist since differing nations have different regulations, such as Candesartan Cilexetil USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Candesartan Cilexetil DMF submitted to regulatory agencies in the US is known as a USDMF. Candesartan Cilexetil USDMF includes data on Candesartan Cilexetil's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Candesartan Cilexetil USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Candesartan Cilexetil suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Candesartan Cilexetil Drug Master File in Japan (Candesartan Cilexetil JDMF) empowers Candesartan Cilexetil API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Candesartan Cilexetil JDMF during the approval evaluation for pharmaceutical products. At the time of Candesartan Cilexetil JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Candesartan Cilexetil suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Candesartan Cilexetil Drug Master File in Korea (Candesartan Cilexetil KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Candesartan Cilexetil. The MFDS reviews the Candesartan Cilexetil KDMF as part of the drug registration process and uses the information provided in the Candesartan Cilexetil KDMF to evaluate the safety and efficacy of the drug.
After submitting a Candesartan Cilexetil KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Candesartan Cilexetil API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Candesartan Cilexetil suppliers with KDMF on PharmaCompass.
A Candesartan Cilexetil CEP of the European Pharmacopoeia monograph is often referred to as a Candesartan Cilexetil Certificate of Suitability (COS). The purpose of a Candesartan Cilexetil CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Candesartan Cilexetil EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Candesartan Cilexetil to their clients by showing that a Candesartan Cilexetil CEP has been issued for it. The manufacturer submits a Candesartan Cilexetil CEP (COS) as part of the market authorization procedure, and it takes on the role of a Candesartan Cilexetil CEP holder for the record. Additionally, the data presented in the Candesartan Cilexetil CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Candesartan Cilexetil DMF.
A Candesartan Cilexetil CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Candesartan Cilexetil CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Candesartan Cilexetil suppliers with CEP (COS) on PharmaCompass.
A Candesartan Cilexetil written confirmation (Candesartan Cilexetil WC) is an official document issued by a regulatory agency to a Candesartan Cilexetil manufacturer, verifying that the manufacturing facility of a Candesartan Cilexetil active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Candesartan Cilexetil APIs or Candesartan Cilexetil finished pharmaceutical products to another nation, regulatory agencies frequently require a Candesartan Cilexetil WC (written confirmation) as part of the regulatory process.
click here to find a list of Candesartan Cilexetil suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Candesartan Cilexetil as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Candesartan Cilexetil API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Candesartan Cilexetil as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Candesartan Cilexetil and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Candesartan Cilexetil NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Candesartan Cilexetil suppliers with NDC on PharmaCompass.
Candesartan Cilexetil Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Candesartan Cilexetil GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Candesartan Cilexetil GMP manufacturer or Candesartan Cilexetil GMP API supplier for your needs.
A Candesartan Cilexetil CoA (Certificate of Analysis) is a formal document that attests to Candesartan Cilexetil's compliance with Candesartan Cilexetil specifications and serves as a tool for batch-level quality control.
Candesartan Cilexetil CoA mostly includes findings from lab analyses of a specific batch. For each Candesartan Cilexetil CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Candesartan Cilexetil may be tested according to a variety of international standards, such as European Pharmacopoeia (Candesartan Cilexetil EP), Candesartan Cilexetil JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Candesartan Cilexetil USP).
